Abstract
As the number of Korean adults with diabetes is expected to increase continuously, the health care for these patients is becoming increasingly important. In recent years, the prevention of infectious diseases through vaccination of the adult population has attracted much interest. Patients with diabetes are more vulnerable to various infectious diseases and the prevention of these diseases through adequate vaccination is especially important. With the recent introduction of newly developed vaccines throughout the country, clinicians have more options for vaccinating their patients. In 2012, the Korean Society of Infectious Diseases developed revised guidelines for adult vaccination, which include recommendations for diabetic patients. Generally, vaccines for healthy adults are also recommended for diabetic patients, and these vaccines include those for Streptococcus pneumoniae, influenza, tetanus-diphtheria-pertussis, hepatitis A and B and herpes zoster. In this review, we will focus our discussion on the pneumococcal (PSV-13 and PPSV 23), influenza and zoster vaccines.
References
1. Oh MD. Adult vaccination: why we need it? In: Vaccination for adults. 2nd ed.Korean Society of Infectious Diseases. Seoul: MIP;2012. p. 2–6.
2. ACIP Adult Immunization Work Group. Bridges CB, Woods L, Coyne-Beasley T. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older–United States, 2013. MMWR Surveill Summ. 2013; 62(Suppl 1):9–19.
3. Korean Diabetes Association, Korea Centers for Disease Control and Prevention. Diabetes fact sheet in Korea, 2012 [Internet]. Seoul: Korean Diabetes Association;2012. [cited 2013 Aug 23]. Available from:. http://www.diabetes.or.kr/temp/Diabetes_Fact_sheet2012.pdf.
4. Lim J, Eom CS, Kim S, Ke S, Cho B. Pneumococcal vaccination rate among elderly in South Korea. J Korean Geriatr Soc. 2010; 14:18–24.

5. OJackson LA, Baxter R, Naleway AL, Belongia EA, Baggs J. Patterns of pneumococcal vaccination and revaccination in elderly and non-elderly adults: a Vaccine Safety Datalink study. BMC Infect Dis. 2009; 9:37.

6. Song JY, Cheong HJ, Heo JY, Noh JY, Seo YB, Kim IS, Choi WS, Kim WJ. Outpatient-based pneumococcal vaccine campaign and survey of perceptions about pneumococcal vaccination in patients and doctors. Yonsei Med J. 2013; 54:469–75.

8. Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, Hospital I, Gomez-Bertomeu F, Raga X. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine. 2009; 27:1504–10.

9. Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, Llor C. EVAN Study Group. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006; 43:860–8.

10. Watson L, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine. 2002; 20:2166–73.

11. Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003; 348:1747–55.

12. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013; 369:155–63.

13. Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, Peters HV, Knutsen B, Hickel J, Parkinson AJ. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55–70 years of age. Clin Infect Dis. 2009; 49:241–8.

14. de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, Gruber WC, Lockhart S, Burkhardt O, Welte T, Lode HM. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis. 2008; 46:1015–23.

15. Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013; 31:3585–93.

16. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, Lu M, So TM, Hsueh PR, Yasin RM, Carlos CC, Pham HV, Lalitha MK, Shimono N, Perera J, Shibl AM, Baek JY, Kang CI, Ko KS, Peck KR. ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012; 56:1418–26.

17. Cho EY, Kang HM, Lee J, Kang JH, Choi EH, Lee HJ. Changes in serotype distribution and antibiotic resistance of nasopharyngeal isolates of Streptococcus pneumoniae from children in Korea, after optional use of the 7-valent conjugate vaccine. J Korean Med Sci. 2012; 27:716–22.

18. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older–United States, 2013. MMWR Surveill Summ. 2013; 62(Suppl 1):1.
19. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011; 378:1962–73.

20. Kee SY, Cheong HJ, Chun BC, Kim WJ. Influenza vaccination coverage rate and factors associated with vaccination in people with chronic disease. Infect Chemother. 2011; 43:406–11.

21. Jimenez-Trujillo I, López-de Andrés A, HernándezBarrera V, Carrasco-Garrido P, Santos-Sancho JM, Jiménez-García R. Influenza vaccination coverage rates among diabetes sufferers, predictors of adherence and time trends from 2003 to 2010 in Spain. Hum Vaccin Immunother. 2013 Feb 12. [Epub].http://dx.doi.org/10.4161/hv.23926.

22. Lau D, Eurich DT, Majumdar SR, Katz A, Johnson JA. Effectiveness of influenza vaccination in working-age adults with diabetes: a population-based cohort study. Thorax. 2013; 68:658–63.

23. Song JY. Adult vaccination: influenza. Vaccination for adults. 2nd ed.Korean Society of Infectious Diseases. Seoul: MIP;2012. p. 140–50.
24. Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008; 57(RR-5):1–30. quiz CE2–4.
25. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005; 352:2271–84.

26. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007; 82:1341–9.

27. Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, Kokia E, Shalev V. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008; 36:226–30.

28. Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013 Jul 16. [Epub].http://dx.doi.org/10.1016/j.jinf.2013.06.016.

29. SOkamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009; 200:1606–10.

30. Satman I, Akalin S, Cakir B, Altinel S, Study Group TD. The effect of physicians' awareness on influenza and pneumococcal vaccination rates and correlates of vaccination in patients with diabetes in Turkey: An epidemiological Study "diaVAX". Hum Vaccin Immunother. 2013 Jul 25. [Epub].http://dx.doi.org/10.4161/hv.25826.

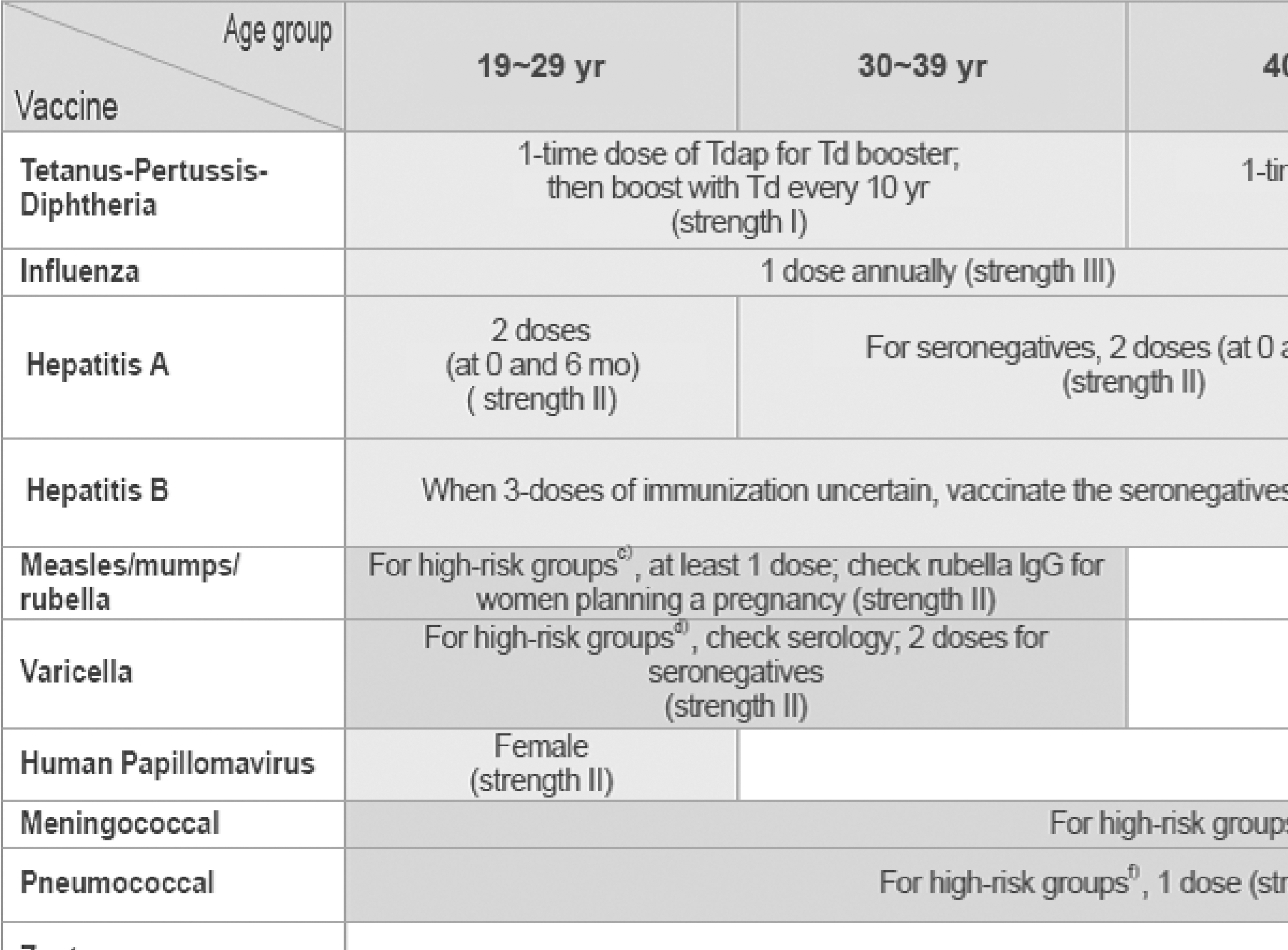
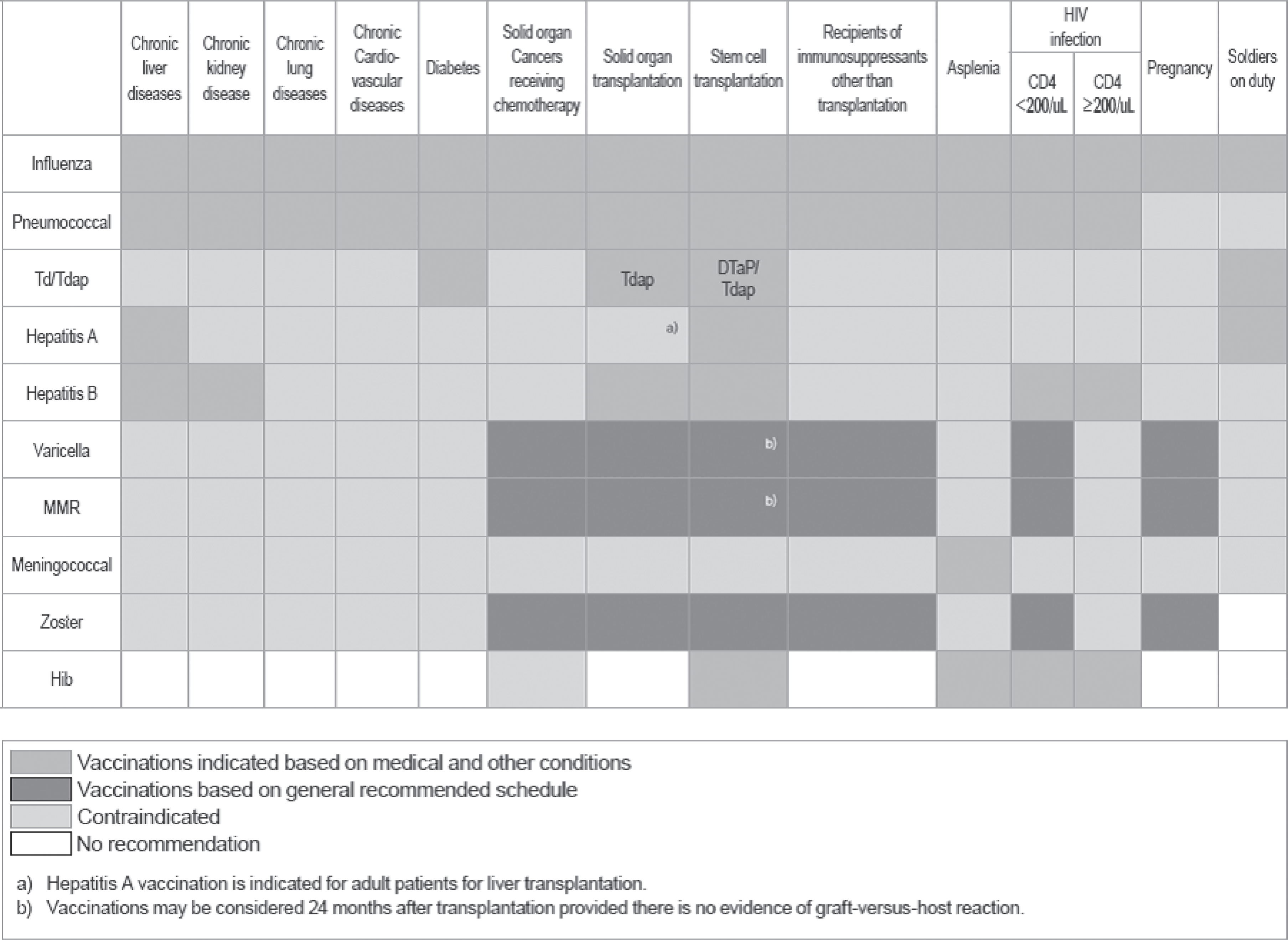
Fig. 1.
Recommended adult immunization schedule, by vaccine and age group (http://www.ksid.or.kr/vaccine_eng.pdf). (A) Hepatitis A (high-risk group): persons with chronic liver disease; persons working at child-care facilities: medical personnel and laboratory workers with potential risk of exposure to the hepatitis A virus; food handlers working at restaurants; persons traveling to or working in countries where hepatitis A is endemic; persons with receive blood products frequently; men who have sex with men; IV drug users; and persons have had contact with acute hepatitis A patients within 2 weeks. (B) Hepatitis B (high-risk group): men who have sex with men; sexually active persons with more than one partner; human immunodeficiency virus (HIV) patients; sexually active persons with more than on partners; human immunodeficiency virus (HIV) patients; IV drug users; household contacts and sexual partners of persons with hepatitis B virus (HBV) carriers; patients with chronic renal failure; patients with chronic liver disease; workers who are frequently exposed to HBV; and clients and staff members of institutions for persons with developmental disabilities. (C) Measles-mumps-rubella (vaccination recommended for high-risk group): Although serological tests (especially for measles) can be done for laboratory evidence of immunity, vaccination without serological tests would be cost saving. High-risk groups: healthcare personnel (serological test required, 2 doses); persons traveling to developing countries; family members who take care of immunocompromised patients; and students who dwell in dormitories. (D) Varicella: vaccination recommended for high-risk group if serologic tests reveal no evidence of immunity. High-risk groups: healthcare workers; family contacts of immunocompromised patients; teachers and child-care employees; students; military personnel; residents of correctional institutions; non-pregnant women expecting pregnancy; adolescents and adults living in households with children; and international travelers. (E) Meningococcal (high-risk group): persons with anatomical of functional asplenia; persons with complement component deficiencies; military personnel (especially for recruits); laboratory workers exposed to meningococcus; persons who travel to or live in an endemic area, particularly if their contact with local populations will be prolonged; and college students living in dormitories. The 2-dose series is recommended for adults with anatomical or functional asplenia, complement component deficiency, and HIV infection; 2 doses should be administered at 0 and 2 months. Revaccinate with meningococcal conjugate vaccine every 5 years for adults who remain at increased risk for infection. (F) Pneumococcal (high-risk group): chronic lung disease (including asthma); chronic cardiovascular disease; diabetes; chronic liver disease; chronic renal failure; nephrotic syndrome; functional or anatomical asplenia; immunocompromised patients (congenital immunodeficiency, HIV infection, leukemia, lymphoma, Hodgkin's disease, multiple myeloma, other malignancy, solid organ transplantation). (vaccinate with 3 or 4 doses of protein conjugate vaccine for hematopoietic stem cell transplants); prolonged use of high-dose corticosteroids or immunosuppressive agents; and cochlear implants. One-time revaccination is recommended for persons aged 65 years or older if they were vaccinated 5 or more years previously and they were less than 65 years of age at the time of primary vaccination. Onetime revaccination after 5 years is recommended for patients with chronic renal failure, nephrotic syndrome, functional or anatomical asplenia, immunocompromised conditions; and prolonged use of immunosuppressive agents.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download