Abstract
Background
Methods
Results
Conclusion
Figures and Tables
Fig. 1
Study design and patient disposition. (A) Schematic of the study design. (B) Details of patient disposition. CON, control group; EXE, exercise group.

Fig. 2
Effects of a combined exercise regimen on the circulating apolipoprotein J (ApoJ) level in postmenopausal women with type 2 diabetes mellitus. (A) Change in the serum ApoJ level over the course of the exercise program. Significant differences in the circulating ApoJ level between the exercise group (EXE) and control group (CON) are shown. (B) Percent changes in the serum ApoJ level, relative to baseline, at weeks 8 and 12. Values are mean±standard error. aP=0.019 at week 8, bP=0.007 at week 12, by repeated-measures analysis of variance, cP<0.05 vs. CON, dP<0.001 vs. CON.

Fig. 3
Correlations of changes in the circulating apolipoprotein J (ApoJ) level with changes in (A) total fat mass (TFM), (B) weight-adjusted appendicular skeletal muscle mass (ASM/wt), and (C) homeostatic model assessment of insulin resistance (HOMA-IR). Data represent Spearman's rank correlation coefficients and P values. Δ indicates the value at baseline minus that at 12 weeks.

Table 1
Metabolic and anthropometric parameters of study participants at baseline and at 12 weeks after intervention week after intervention

Values are presented as mean±standard deviation and median (interquartile range).
EXE, exercise group; CON, control group; NA, not applicable; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; eGFR, estimated glomerular filtration rate; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; CRP, C-reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance; ApoJ, apolipoprotein J; ACR, albumin-to-creatinine ratio; WC, waist circumference; BMI, body mass index; ASM, appendicular skeletal muscle mass; wt, weight.
aP>0.05 by two sample t-test or Mann-Whitney U test between two groups at baseline, bPaired t-test or Wilcoxon signed ranks test for within group, cTwo-way repeated measures analysis of variance over time for between groups, dLogarithm-transformed values were used for comparison.
Table 2
Correlations of ΔApoJ with changes in other metabolic and anthropometric parameters at the end of the 12 weeks after combined exercise
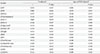
Data represent Spearman's rank correlation coefficient values and P values. Δ indicates data at baseline minus data at 12 weeks.
ApoJ, apolipoprotein J; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; WC, waist circumference; ASM, appendicular skeletal muscle mass; wt, weight.
aOnly age (not BMI) adjusted in anthropometric parameters, bLogarithm-transformed values were used for comparison.
Notes
CONFLICTS OF INTEREST This work was supported by grants from the National Institutes of Health (R01DK111529 and R01DK106076 to Young-Bum Kim), R&D project grant from Yuhan pharmaceutical corporation (to Sang Soo Kim) and National Research Foundation of Korea (2018R1C1B6002854 to Sang Soo Kim).
AUTHOR CONTRIBUTION
Conception or design: Y.K.J., S.S.K., I.J.K., Y.B.K.
Acquisition, analysis, or interpretation of data: Y.K.J., S.S.K., J.H.K., H.J.K., H.J.K., J.J.P., Y.S.C., S.H.J., J.R.K., B.H.K., S.H.S., I.J.K., Y.K.K., Y.B.K.
Drafting the work or revising: Y.K.J., S.S.K., Y.B.K.
Final approval of the manuscript: Y.K.J., S.S.K., J.H.K., H.J.K., H.J.K., J.J.P., Y.S.C., S.H.J., J.R.K., B.H.K., S.H.S., I.J.K., Y.K.K., Y.B.K.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download