1. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008; 28:629–636. PMID:
18174459.
2. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 56:1113–1132. PMID:
20863953.
3. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007; 49:403–414. PMID:
17258085.
4. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–1645. PMID:
19805654.
5. Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017; 49:e291. PMID:
28154371.

6. Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005; 280:847–851. PMID:
15509553.

7. Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016; 65:1049–1061. PMID:
26997538.

8. Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis. 2010; 20:72–77. PMID:
19747805.

9. Mandl J, Meszaros T, Banhegyi G, Hunyady L, Csala M. Endoplasmic reticulum: nutrient sensor in physiology and pathology. Trends Endocrinol Metab. 2009; 20:194–201. PMID:
19349192.

10. Oike Y, Ito Y, Maekawa H, Morisada T, Kubota Y, Akao M, Urano T, Yasunaga K, Suda T. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood. 2004; 103:3760–3765. PMID:
14764539.

11. Oike Y, Yasunaga K, Ito Y, Matsumoto S, Maekawa H, Morisada T, Arai F, Nakagata N, Takeya M, Masuho Y, Suda T. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc Natl Acad Sci U S A. 2003; 100:9494–9499. PMID:
12871997.

12. Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, Urano T, Kimura Y, Kubota Y, Maekawa H, Miyamoto T, Miyata K, Matsumoto S, Sakai J, Nakagata N, Takeya M, Koseki H, Ogawa Y, Kadowaki T, Suda T. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005; 11:400–408. PMID:
15778720.

13. Kitazawa M, Ohizumi Y, Oike Y, Hishinuma T, Hashimoto S. Angiopoietin-related growth factor suppresses gluconeogenesis through the Akt/forkhead box class O1-dependent pathway in hepatocytes. J Pharmacol Exp Ther. 2007; 323:787–793. PMID:
17804676.

14. Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med. 2005; 11:473–479. PMID:
16154386.

15. Stepan H, Ebert T, Schrey S, Reisenbuchler C, Stein S, Lossner U, Bluher M, Stumvoll M, Kratzsch J, Faber R, Fasshauer M. Serum levels of angiopoietin-related growth factor are increased in preeclampsia. Am J Hypertens. 2009; 22:314–318. PMID:
19057519.

16. Boztosun A, Deveci K, Klcl F, Soylemez MS, Muhtaroglu S, Muderris II. Serum levels of angiopoietin-related growth factor (AGF) are increased in polycystic ovary syndrome. J Investig Med. 2012; 60:813–817.

17. Ebert T, Bachmann A, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of angiopoietin-related growth factor in diabetes mellitus and chronic hemodialysis. Metabolism. 2009; 58:547–551. PMID:
19303977.

18. Ebert T, Kralisch S, Loessner U, Jessnitzer B, Stumvoll M, Fasshauer M, Tonjes A. Relationship between serum levels of angiopoietin-related growth factor and metabolic risk factors. Horm Metab Res. 2014; 46:685–690. PMID:
25011017.

19. Namkung J, Koh SB, Kong ID, Choi JW, Yeh BI. Serum levels of angiopoietin-related growth factor are increased in metabolic syndrome. Metabolism. 2011; 60:564–568. PMID:
20673930.

20. Kim JY, Ahn SV, Yoon JH, Koh SB, Yoon J, Yoo BS, Lee SH, Park JK, Choe KH, Guallar E. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes Care. 2013; 36:1547–1553. PMID:
23275369.
21. Choi JR, Kim JY, Park IH, Huh JH, Kim KW, Cha SK, Park KS, Sohn JH, Park JT, Koh SB. Serum fibroblast growth factor 21 and new-onset metabolic syndrome: KoGES-ARIRANG Study. Yonsei Med J. 2018; 59:287–293. PMID:
29436198.

22. Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007; 75:72–80. PMID:
16735075.

23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID:
3203132.

24. Galletti F, Barbato A, Versiero M, Iacone R, Russo O, Barba G, Siani A, Cappuccio FP, Farinaro E, della Valle E, Strazzullo P. Circulating leptin levels predict the development of metabolic syndrome in middle-aged men: an 8-year follow-up study. J Hypertens. 2007; 25:1671–1677. PMID:
17620965.

25. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004; 110:380–385. PMID:
15262834.

26. Mora S, Pessin JE. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab Res Rev. 2002; 18:345–356. PMID:
12397577.

27. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002; 3:141–146. PMID:
12164465.

28. Wang D, Li Y, Lee SG, Wang L, Fan J, Zhang G, Wu J, Ji Y, Li S. Ethnic differences in body composition and obesity related risk factors: study in Chinese and white males living in China. PLoS One. 2011; 6:e19835. PMID:
21625549.

29. Roh E, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Kim DJ, Choi SH, Lim S, Cha BY. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Prevalence and management of dyslipidemia in Korea: Korea National Health and Nutrition Examination Survey during 1998 to 2010. Diabetes Metab J. 2013; 37:433–449. PMID:
24404515.

30. Kim SM, Han JH, Park HS. Prevalence of low HDL-cholesterol levels and associated factors among Koreans. Circ J. 2006; 70:820–826. PMID:
16799232.

31. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011; 34:1323–1328. PMID:
21505206.
32. Hong AR, Lim S. Clinical characteristics of metabolic syndrome in Korea, and its comparison with other Asian countries. J Diabetes Investig. 2015; 6:508–515.
33. Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim DH, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013; 19:83–92. PMID:
23202295.

34. Kim MJ, Namkung J, Chang JS, Kim SJ, Park KS, Kong ID. Leptin regulates the expression of angiopoietin-like 6. Biochem Biophys Res Commun. 2018; 502:397–402. PMID:
29852166.

35. Kang SG, Yi HS, Choi MJ, Ryu MJ, Jung S, Chung HK, Chang JY, Kim YK, Lee SE, Kim HW, Choi H, Kim DS, Lee JH, Kim KS, Kim HJ, Lee CH, Oike Y, Shong M. ANGPTL6 expression is coupled with mitochondrial OXPHOS function to regulate adipose FGF21. J Endocrinol. 2017; 233:105–118. PMID:
28184000.

36. Ricci PF, Straja SR, Cox AL Jr. Changing the risk paradigms can be good for our health: J-shaped, linear and threshold dose-response models. Dose Response. 2012; 10:177–189. PMID:
22740780.

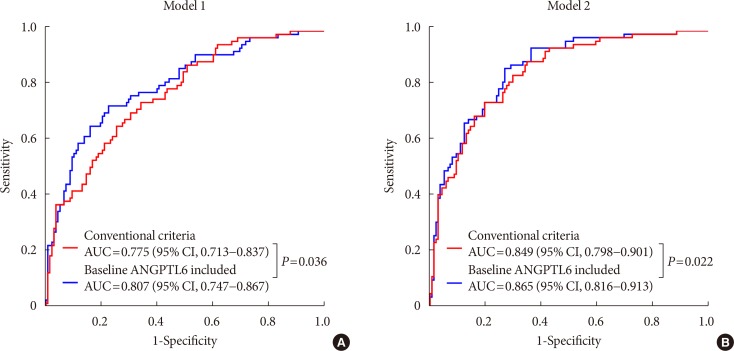
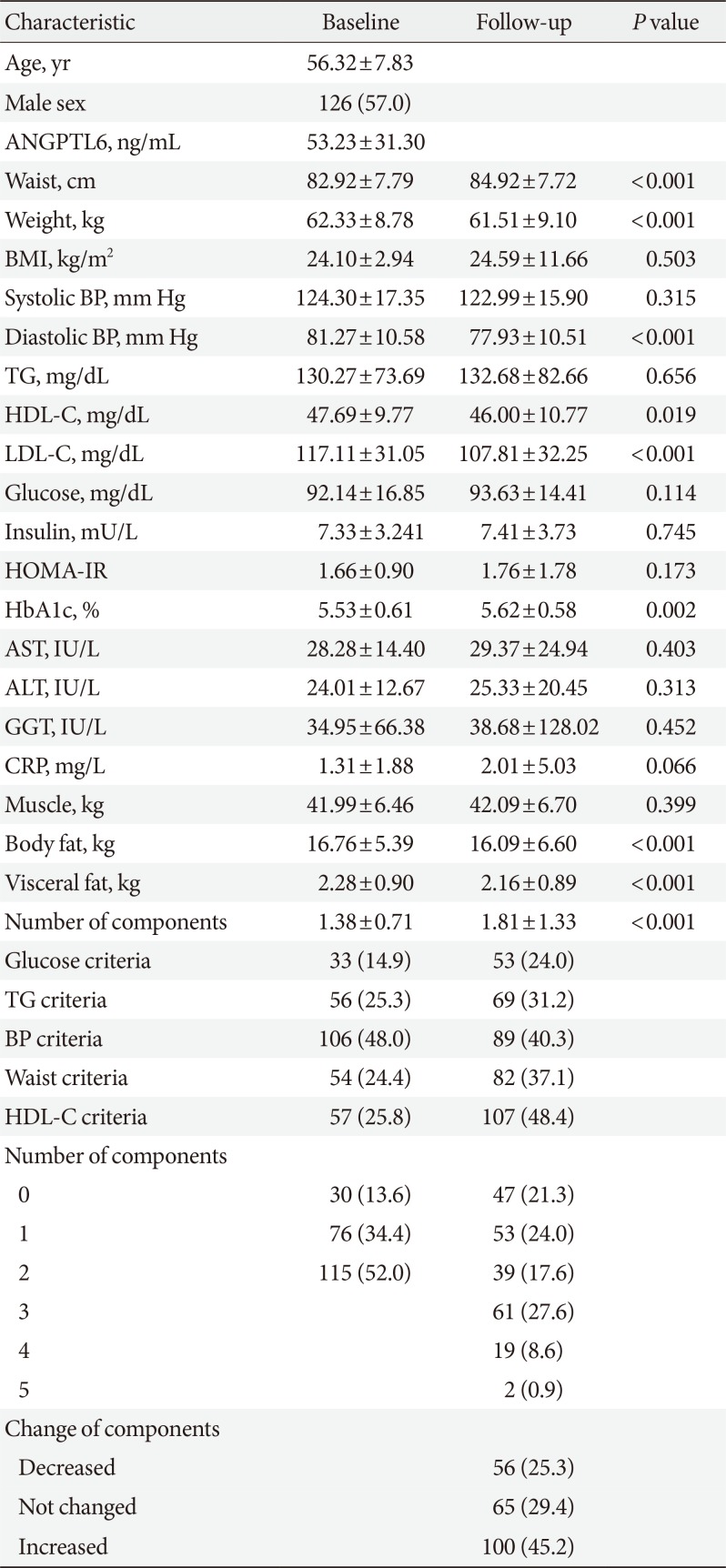
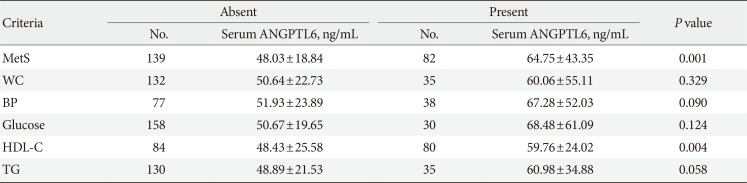
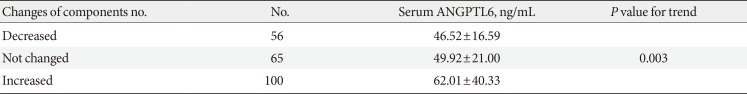




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download