Abstract
Background
Methods
Results
References
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Table 4
Supplementary Table 5
Supplementary Fig. 1
Fig. 1
Selection of study participants. MetS, metabolic syndrome; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
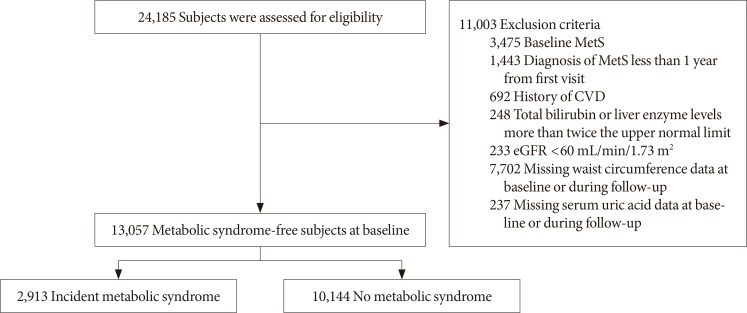
Table 1
Baseline characteristics for both sexes according to development of metabolic syndrome
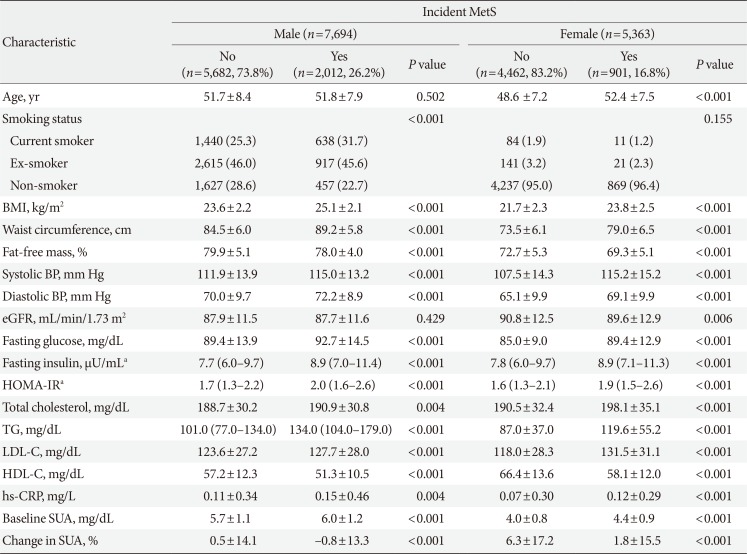
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
MetS, metabolic syndrome; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HOMA-IR, homeostasis model assessment index for insulin resistance; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; SUA, serum uric acid.
an=5,188 male, n=2,792 female.
Table 2
Baseline clinical and biochemical characteristic of study subjects based on percent change in serum uric acid levels quartile categories and sex
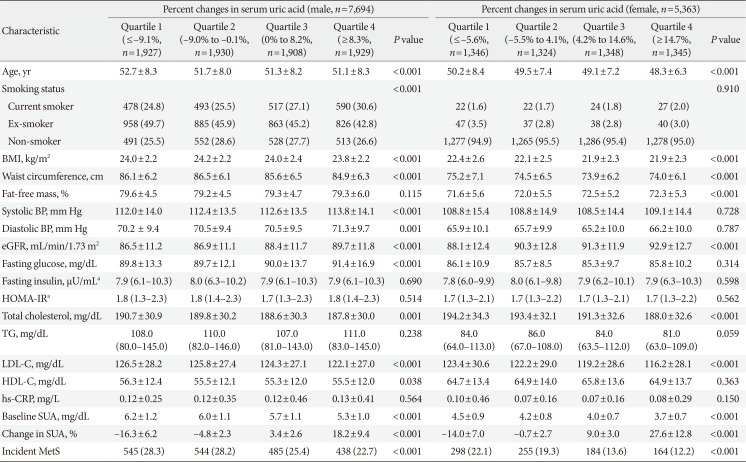
Values are presented as mean±standard deviation, number (%), or median (interquartile range). Characteristics of the study population according to the serum uric acid quartile were compared using one-way analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables and chi-square test for categorical variables.
MetS, metabolic syndrome; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HOMA-IR, homeostasis model assessment index for insulin resistance; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; SUA, serum uric acid.
an=5,188 male, n=2,792 female.
Table 3
Correlations between serum uric acid levels, percent changes in serum uric acid and metabolic parameters according to both sexes
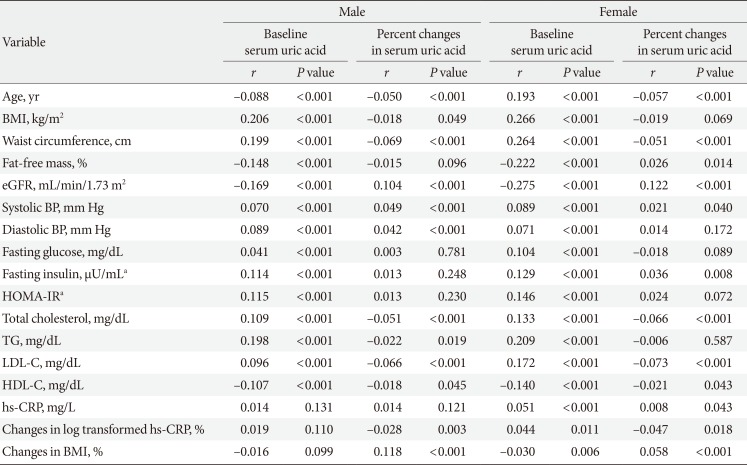
CRP was log transformed to meet the demands of normal distribution.
BMI, body mass index; eGFR, estimated glomerular filtration rate; BP, blood pressure; HOMA-IR, homeostasis model assessment index for insulin resistance; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
an=5,188 male, n=2,792 female.
Table 4
Hazard ratios and 95% confidence intervals for development of metabolic syndrome according to quartile of percent change in serum uric acid levels: male
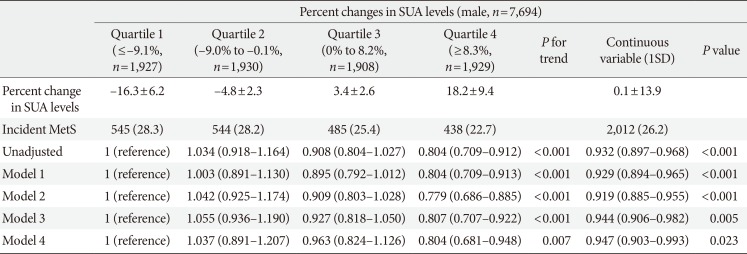
Values are presented as mean±standard deviation, number (%), hazard ratio (95% confidence interval). Model 1: adjusted for age, systolic blood pressure, body mass index, fat-free mass (%), estimated glomerular filtration rate, and smoking status; Model 2: adjusted for Model 1 plus fasting glucose, triglyceride, low density lipoprotein cholesterol, and high density lipoprotein cholesterol; Model 3: adjusted for Model 2 plus baseline SUA; Model 4: adjusted for Model 3 plus fasting insulin.a
UA, serum uric acid; SD, standard deviation; MetS, metabolic syndrome.
an=5,188 male.
Table 5
Hazard ratios and 95% confidence intervals for development of metabolic syndrome according to quartile of percent change in serum uric acid levels: female
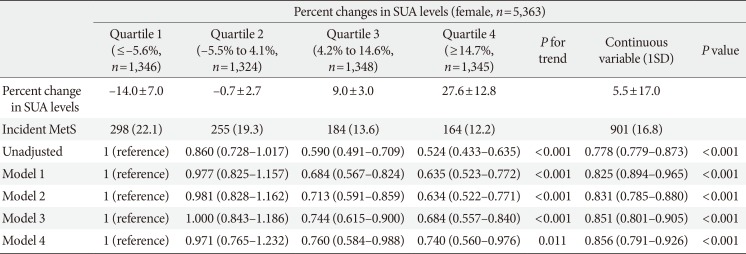
Values are presented as mean±standard deviation, number (%), or hazard ratio (95% confidence interval). Model 1: adjusted for age, systolic blood pressure, body mass index, fat-free mass (%), estimated glomerular filtration rate, and smoking status; Model 2: adjusted for Model 1 plus fasting glucose, triglyceride, low density lipoprotein cholesterol, and high density lipoprotein cholesterol; Model 3: adjusted for Model 2 plus baseline SUA; Model 4: adjusted for Model 3 plus fasting insulin.a
SUA, serum uric acid; SD, standard deviation; MetS, metabolic syndrome.
an=2,792 female.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download