Abstract
Background
Glycosylated hemoglobin (HbA1c) has been recommended as a diagnostic test for prediabetes and diabetes. Here, we evaluated the level of agreement between diagnoses based on fasting plasma glucose (FPG) versus HbA1c levels and determined optimal HbA1c cutoff values for these diseases in youth and young adults.
Methods
The study included 7,332 subjects (n=4,129, aged 10 to 19 years in youth group; and n=3,203 aged 20 to 29 years in young adult group) from the 2011 to 2016 Korea National Health and Nutrition Examination Survey. Prediabetes and diabetes were defined as 100 to 125 mg/dL (impaired fasting glucose [IFG]) and ≥126 mg/dL for FPG (diabetes mellitus [DM] by FPG [DMFPG]), and 5.7% to 6.4% and ≥6.5% for HbA1c, respectively.
Results
In the youth group, 32.5% with IFG had an HbA1c level of 5.7% to 6.4%, and 72.2% with DMFPG had an HbA1c ≥6.5%. In the young adult group, 27.5% with IFG had an HbA1c level of 5.7% to 6.4%, and 66.6% with DMFPG had an HbA1c ≥6.5%. Kappa coefficients for agreement between the FPG and HbA1c results were 0.12 for the youth group and 0.19 for the young adult group. In receiver operating characteristic curve analysis, the optimal HbA1c cutoff for IFG and DMFPG were 5.6% and 5.9% in youths and 5.5% and 5.8% in young adults, respectively.
The prevalence of obesity and type 2 diabetes mellitus (DM) is increasing worldwide among adolescents and young adults [1]. Impaired fasting glucose (IFG) and impaired glucose tolerance (collectively known as prediabetes) precede diabetes [23], and prediabetes is more prevalent in these age groups than is diabetes [4]. Children and adolescents with elevated fasting plasma glucose (FPG) have been predicted to have type 2 DM in young adulthood [5].
The diagnosis of diabetes and prediabetes has been traditionally based on the FPG and 2-hour plasma glucose levels in oral glucose tolerance tests (OGTTs). However, an International Expert Committee has recommended measurement of glycosylated hemoglobin (HbA1c) levels as the diagnostic method, with cutoff values of ≥6.5% for diabetes and ≥6.0% for high risk for diabetes [6]. The value for prediabetes recommended by the American Diabetes Association (ADA) is ≥5.7% [7]. The Korean Diabetes Association (KDA) has included HbA1c as a diagnostic test since 2011 [8].
HbA1c is a non-enzymatic glycosylated product of hemoglobin, and its level reflects the mean glucose concentration in the blood for the preceding 3 to 4 months. A high level of HbA1c is a risk factor for diabetes and diabetes-related complications according to epidemiologic studies [91011]. Measurement of HbA1c levels has several advantages over measurement of FPG or 2-hour plasma glucose levels, which include no need for fasting, less inter-individual variability, and better biologic stability after sampling [612]. Although HbA1c has been used as a tool for detecting diabetes and prediabetes, diagnoses based on HbA1c levels are not always the same as those based on glucose levels [131415]. Studies performed in obese Korean children and adolescents showed discordance between OGTT- and HbA1c-based results in predicting diabetes and prediabetes [1617]. Several studies have revealed the differences in normative HbA1c values with respect to age, sex, and ethnicity [1819]. Whether the HbA1c cutoff values for diabetes and prediabetes should be the same in adults versus adolescents is controversial [202122]. Moreover, studies for the usefulness of HbA1c in detecting prediabetes and diabetes based on FPG in both general pediatric and young adult populations are lacking.
The aims of the present study were to assess the extent of agreement between diagnoses based on FPG versus HbA1c levels, to evaluate the diagnostic performance of HbA1c, and to determine the optimal HbA1c cutoff values for diabetes and prediabetes in youths and young adults by using nationally representative data of Korea.
The data for the study were obtained from the Korea National Health and Nutrition Examination Survey (KNHANES) performed between 2011 and 2016. The KNHANES was a nationally representative cross-sectional examination of non-institutionalized Korean citizens with a multi-stage clustered probability design conducted by the Korea Centers for Disease Control and Prevention [23]. This survey aimed to assess the health and nutrition of Koreans, to monitor risk factors for major chronic diseases, and to provide reliable data for health policies [23]. The detailed methods for data collection by the KNHANES are described elsewhere [24].
A total of 47,464 individuals were enrolled in the KNHANES from 2011 to 2016. For this study, 9,502 subjects aged 10 to 29 years were considered as potential participants. Among them, 7,332 were included in the study after exclusion of participants who had fasted <8 hours before sample collection (n=310), had no glucose or HbA1c data (n=1,647), were previously diagnosed with DM (n=11), were pregnant at the time of the survey (n=53), or had anemia with a hemoglobin level <11.5 g/dL in children aged 10 to 11 years, <12 g/dL in children aged 12 to 14 years and females aged ≥15 years, or <13 g/dL for males aged ≥15 years (n=343) (Fig. 1) [25]. Subjects with hemoglobinopathy were not considered for the present study, because the condition is extremely rare in the Korean population [26]. Among the 7,332 participants in the 2011 to 2016 KNHANES who met the inclusion criteria, 4,129 (45.1%) were in the youth group (10 to 19 years of age) and 3,203 (54.9%) were in the young adult group (20 to 29 years of age).
Subjects were divided into two groups according to age: the youth group (10 to 19 years of age) and the young adult group (20 to 29 years of age). In this study, DM by FPG (DMFPG) was defined as an FPG level ≥126 mg/dL, and IFG was defined as an FPG level between 100 and 125 mg/dL. We used the HbA1c cutoff criteria recommended by the ADA and KDA, namely, ≥6.5% for DM by HbA1c (DMA1C) and 5.7% to 6.4% for prediabetes by HbA1c (PreDMA1C) [78].
All subjects participated in the KNHANES voluntarily and provided informed consent. The protocol of the KNHANES was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (IRB No. 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C) [2324]. The present study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. X-1703-388-912). All procedures were performed in accordance with the Declaration of Helsinki.
Blood samples were drawn after overnight fasting by trained medical personnel. The samples were transported daily to the central laboratory and analyzed within 24 hours. FPG levels were measured by using the hexokinase method with a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). HbA1c levels were measured via high performance liquid chromatography (HLC-723G7; Tosoh, Tokyo, Japan), which is the method certified by the National Glycohemoglobin Standardization Program [27]. The laboratory tests performed in the KNHANES underwent both internal and external quality controls three times a year. The detailed methods for anthropometric measurements and laboratory tests are described elsewhere [24].
Statistical analyses were performed by using Stata version 14.2 software (StataCorp LP, College Station, TX, USA). Appropriate sample weights were used to adjust for the multi-stage clustered probability sample design. Taylor series linearization was used for variance estimation.
Results were expressed as weighted mean±standard error or number (weighted percent). Kappa coefficients were calculated to assess agreement between FPG and HbA1c results for the diagnosis of prediabetes and diabetes [28]. The diagnostic performance of HbA1c was evaluated in terms of sensitivity, specificity, positive predictive value (PPV), and negative predictive value at thresholds of 5.7% and 6.5%. Receiver operating characteristic (ROC) curves were generated to assess the ability of HbA1c to predict IFG and DMFPG [29]. The equality of area under the curve (AUC) with a 95% confidence interval (CI) was evaluated. The optimal cutoff values were the points at which the ROC curve was closest to the point at which sensitivity and specificity were equal to 1. A P value <0.05 was considered statistically significant.
There was no sex difference found between the groups (males 54.6% for the youth group and 55.8% for the young adult group) (Supplementary Table 1). FPG and HbA1c levels were significantly higher in the youth group than in the young adults group (FPG 90.3±0.2 and 89.1±0.2 mg/dL; HbA1c 5.41%± 0.01% and 5.32%±0.01%, respectively). The values of all variables except for FPG and HbA1c levels were significantly higher in the young adult group than in the youth group (Supplementary Table 1).
In the youth group, HbA1c levels were as follows: 5.7% to 6.4% in 110 (32.5%) of the participants with IFG; and ≥6.5% in five (72.2%) of the participants with DMFPG (Table 1). In the young adult group, the HbA1c levels were as follows: 5.7% to 6.4% in 54 (27.5%) of the participants with IFG and ≥6.5% in 12 (66.6%) of the participants with DMFPG. The kappa coefficients for agreement between the FPG and HbA1c results were 0.12 in the youth group and 0.19 in the young adult group, which indicated poor agreement [28].
When an HbA1c cutoff of 5.7% was used to detect IFG, the sensitivity and specificity were 35.0% and 83.9% in the youth group and 33.2% and 90.9% in the young adult group, respectively (Table 2). When an HbA1c cutoff of 6.5% was used to detect DMFPG, the sensitivity and specificity were 72.2% and 99.9% in the youth group and 66.6% and 99.9% in the young adult group, respectively.
In the ROC curve analysis, the AUC (95% CI) for detecting IFG based on HbA1c level was 0.649 (95% CI, 0.648 to 0.650) for the youth group and 0.700 (95% CI, 0.699 to 0.701) for the young adult group (P<0.001) (Fig. 2A). The optimal HbA1c cutoff point for diagnosing IFG was 5.6% (sensitivity 49.9%, specificity 73.3%) in the youth group and 5.5% (sensitivity 54.3%, specificity 72.9%) in the young adult group (Table 2).
The AUC (95% CI) for detecting DMFPG based on HbA1c level was 0.996 (95% CI, 0.996 to 0.996) for the youth group and 0.962 (95% CI, 0.962 to 0.963) for the young adult group (P<0.001) (Fig. 2B). The optimal HbA1c cutoff point for diagnosing DMFPG was 5.9% (sensitivity 100%, specificity 95.8%) in the youth group and 5.8% (sensitivity 87.9%, specificity 94.6%) in the young adult group (Table 2).
By using nationally representative survey data, we assessed the diagnostic performance of HbA1c cutoff values recommended for the diagnosis of IFG and DMFPG. Use of an HbA1c cutoff of ≥6.5% for DMFPG resulted in a sensitivity of 72.2% and a specificity of 99.9% in the youth group and a sensitivity of 66.6% and a specificity of 99.9% in the young adult group. However, the HbA1c cutoff of ≥5.7% for IFG had a lower sensitivity and specificity than did the HbA1c cutoff of 6.5% for diabetes in both groups. In the present study, the HbA1c cutoff values that best coincided with the DMFPG were 5.9% in the youth group and 5.8% in the young adult group by using ROC curve analysis. The optimal HbA1c cutoff levels for detecting IFG in the youth and young adult groups were 5.6% and 5.5%, respectively.
Collectively, the data presented above support the use of different HbA1c thresholds for identifying IFG and DMFPG in pediatric and young adult populations. The cutoff values of HbA1c predicting IFG and DMFPG were lower than those recommended by the ADA in the adult population.
In this study population, when HbA1c alone was used as a tool for detecting DMFPG, only three youths (0.07%) and five young adults (0.12%) with DMFPG were misdiagnosed with PreDMA1C. However, for the diagnosis of IFG using HbA1c, 214 youths (5.3%) and 149 young adults (4.9%) with IFG were misclassified as being normoglycemic (Table 1). According to the present study, an HbA1c of 6.5% might play a role in confirming the diagnosis of diabetes in the pediatric population because of high specificity and PPV (Table 2). This suggests that HbA1c alone might be insufficient to diagnose IFG in youths and young adults.
When applying the HbA1c cutoff value of 5.6% for IFG in the youth group, the misclassification rate decreased to 4.1% (163 subjects). In the young adult group, applying the HbA1c cutoff of 5.5% to detect IFG decreased misclassification rate to 3.4% (103 subjects). Therefore, in these age groups, lower HbA1c cutoff might be used to detect subjects at risk for development of diabetes.
After adopting HbA1c as a diagnostic test for DM, validation studies were performed in adult and pediatric populations (Table 3) [16172230313233]. The cutoff values of HbA1c for detecting prediabetes and diabetes were found not to be the same as the values recommended by the ADA.
The HbA1c threshold for prediabetes and diabetes might be associated with the HbA1c levels of a certain population. Populations with higher mean HbA1c levels seemed to show higher HbA1c cutoff values for prediabetes and diabetes. In the present study, young adults aged 20 to 29 years had lower HbA1c cutoff points for IFG and DMFPG than did youths aged 10 to 19 years. Generally, HbA1c levels increase with age [34]. HbA1c thresholds for prediabetes and diabetes also increase with age [30]. However, several studies have revealed that teenagers have higher HbA1c levels than adults in their twenties [1819]. In the general Korean population, mean HbA1c levels were 5.42% for youths aged 10 to 19 years and 5.32% for young adults aged 20 to 29 years [18]. Moreover, Korean youths and young adults showed higher HbA1c levels than did their counterparts in the United States (5.37% from Korea versus 4.99% from the United States) [1819]. HbA1c cutoff points of prediabetes and diabetes in the United States pediatric population was 5.5% and 5.8%, respectively, which was lower than those in Korean youths [22].
In the adult population aged >30 years, FPG usage, as compared with HbA1c usage, has been found to underestimate diabetes and prediabetes in Korean and Chinese studies [153536]. However, studies of primarily Caucasians and African Americans in the United States have reported to opposite results [1121]. Ethnic variations in HbA1c levels might be the reason for these results; e.g., Asians have higher HbA1c levels than African Americans and Caucasians [1819]. In the present study, HbA1c alone underestimated IFG and DMFPG in subjects aged <30 years (Table 1). This suggests that additional evaluation by using FPG to detect diabetes and prediabetes might be warranted in those aged <30 years with lower HbA1c values.
Interestingly, cutoff points for prediabetes and diabetes in the present study corresponded to the 75th and 95th percentiles for both age groups according to recently published Korean reference values for HbA1c (5.6% for the 75th percentile and 5.9% for the 95th percentile in the youth group; 5.5% for the 75th percentile and 5.8% for the 95th percentile in the young adult group) [18]. This implies that an HbA1c value of the 75th percentile among those aged <30 years might be an appropriate cutoff, rather than the uniform value provided by the ADA. An HbA1c of 6.5%, which was the diagnostic criteria for diabetes, far exceeded over the 99th percentile in the Korean population aged <30 years.
HbA1c is useful for predicting DM and cardiovascular complications [11]. However, there are discrepancies in the diagnoses of diabetes and prediabetes based on HbA1c versus FPG levels. In the present study, the kappa coefficients indicated poor agreement between HbA1c and FPG results in both groups (0.12 for the youth group and 0.19 for the young adult group). Similar to our findings, others have reported a kappa coefficient of 0.17 for agreement between HbA1c results and 2-hour plasma glucose results of OGTTs for diagnosis of DM in obese children and adolescents [22]. Therefore, HbA1c criteria for the diagnosis of prediabetes and diabetes might be different in children and adults. Glucose- and HbA1c-based test might be complementary in detecting prediabetes and diabetes. Moreover, this approach might help to detect early changes in abnormal glucose metabolism.
In the present study, IFG and DMFPG was defined by FPG levels due to the lack of 2-hour plasma glucose after OGTT. However, FPG elevation within the normal glycemic range in childhood was found to be a reliable marker for future development of diabetes in young adulthood [5]. This result suggests the importance of FPG levels in the pediatric population.
Several factors may influence the variance of HbA1c levels, including the hemoglobin glycosylation rate and the rate of turnover of red blood cells [373839]. Although we did not evaluate these parameters in this study, we excluded participants with anemia, because iron deficiency has been shown to elevate HbA1c levels.
This study had several limitations. First, only a single fasting sample was examined, which might have caused misclassification bias. Second, OGTT were not performed. Diabetes could be diagnosed based on either FPG or 2-hour plasma glucose. Moreover, variance in HbA1c is affected by both fasting and postprandial glucose. However, in a large-scale survey such as the KNHANES, OGTT could be difficult to apply. Third, because of the cross-sectional study design, whether a current HbA1c value could predict diabetes and its related complications is unclear. In addition, HbA1c could not indicate acute glucose fluctuation or variation. Fourth, the low number of patients with diabetes in the youth group might have affected the statistical significance (DMFPG 0.23%, DMA1C 0.23% in the youth group; DMFPG 0.58%, DMA1C 0.46% in the young adult group). The strength of the present study is the comparison between the diagnostic performance of HbA1c in youth and young adult groups by using nationally representative data.
In summary, although HbA1c has been recommended as a diagnostic marker for diabetes and prediabetes in adults, its usefulness in children and adolescents remains unclear owing to the disparity between the results of glucose- and HbA1c-based tests. Lower optimal HbA1c cutoff values for the diagnosis of IFG and DMFPG were observed in youths and young adults as compared with those recommended for adults. Therefore, a lower HbA1c level for the detection of prediabetes and diabetes, as compared to that recommended by the ADA, might be more appropriately applied to the Korean population aged <30 years. Prospective studies that include OGTTs are needed to clarify the diagnostic role of HbA1c in both diabetes and prediabetes in pediatric and young adult populations.
References
1. Cali AM, Caprio S. Prediabetes and type 2 diabetes in youth: an emerging epidemic disease? Curr Opin Endocrinol Diabetes Obes. 2008; 15:123–127. PMID: 18316946.

2. Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002; 19:708–723. PMID: 12207806.

3. Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, Yazdi H, Booker L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312. PMID: 17601626.

4. Ek AE, Rossner SM, Hagman E, Marcus C. High prevalence of prediabetes in a Swedish cohort of severely obese children. Pediatr Diabetes. 2015; 16:117–128. PMID: 24635861.

5. Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: the Bogalusa Heart Study. Arch Pediatr Adolesc Med. 2010; 164:124–128. PMID: 20124140.

6. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009; 32:1327–1334. PMID: 19502545.
7. American Diabetes Association. Standards of medical care in diabetes: 2010. Diabetes Care. 2010; 33(Suppl 1):S11–S61. PMID: 20042772.
8. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, Woo MH, Kim JY, Kim NH, Kim JT, Kim CH, Kim HJ, Jeong IK, Hong EK, Cho JH, Mok JO, Yoon KH; Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2011 Clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011; 35:431–436. PMID: 22111032.

9. Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Utility of hemoglobin A1c in predicting diabetes risk. J Gen Intern Med. 2004; 19:1175–1180. PMID: 15610327.

10. Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007; 120:720–727. PMID: 17679132.

11. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010; 362:800–811. PMID: 20200384.

12. Bloomgarden ZT. A1C: recommendations, debates, and questions. Diabetes Care. 2009; 32:e141–e147. PMID: 19940210.

13. Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010; 33:562–568. PMID: 20067953.

14. Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care. 2010; 33:2190–2195. PMID: 20628087.

15. Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Lim S, Choi SH, Jang MJ, Kim Y, Oh K, Kim DJ, Cha BY. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J. 2013; 37:349–357. PMID: 24199164.

16. Lee HS, Park HK, Hwang JS. HbA1c and glucose intolerance in obese children and adolescents. Diabet Med. 2012; 29:e102–e105. PMID: 22273110.
17. Nam HK, Cho WK, Kim JH, Rhie YJ, Chung S, Lee KH, Suh BK. HbA1c cutoff for prediabetes and diabetes based on oral glucose tolerance test in obese children and adolescents. J Korean Med Sci. 2018; 33:e93. PMID: 29542302.

18. Seo JY, Hwang SS, Kim JH, Lee YA, Lee SY, Shin CH, Yang SW. Distribution of glycated haemoglobin and its determinants in Korean youth and young adults: a nationwide population-based study. Sci Rep. 2018; 8:1962. PMID: 29386645.

19. Saaddine JB, Fagot-Campagna A, Rolka D, Narayan KM, Geiss L, Eberhardt M, Flegal KM. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care. 2002; 25:1326–1330. PMID: 12145229.
20. Shah S, Kublaoui BM, Oden JD, White PC. Screening for type 2 diabetes in obese youth. Pediatrics. 2009; 124:573–579. PMID: 19620188.

21. Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011; 158:947–952. PMID: 21195416.

22. Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, Guandalini C, Savoye M, Rose P, Caprio S. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011; 34:1306–1311. PMID: 21515842.

23. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77. PMID: 24585853.

24. Ministry of Health and Welfare. The Korea National Health and Nutritional Examination Survey. cited 2018 Oct 23. Available from: https://knhanes.cdc.go.kr/knhanes/main.do.
25. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity 2011 (WHO/NMH/NHD/MNM/11.1). cited 2018 Oct 23. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
26. Yang SE, Park CJ, Nah J, Min WK, Chi HS. Hematologic characteristics and hemoglobin fraction analysis by high performance liquid chromatogaphy in patients with hypochromic microcytosis: trials for detection of beta-thalassemia. Korean J Lab Med. 2005; 25:145–151.
27. The National Glycohemoglobin Standardization Program. List of NGSP certified methods. cited 2018 Oct 23. Available from: http://www.ngsp.org/docs/methods.pdf.
28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.

29. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007; 96:644–647. PMID: 17376185.

30. Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, Moon S, Park IeB, Rhee EJ, Chung CH, Kim BJ, Ku BJ. Optimal hemoglobin A1C cutoff value for diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013; 99:231–236. PMID: 23541039.

31. Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, Wu P, Teng X, Dong Y, Zhou Jw, Xu H, Zheng J, Li S, Tao T, Hu Y, Jia Y. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 2010; 47:231–236. PMID: 19760291.

32. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med. 2007; 24:333–343. PMID: 17367307.
33. Hosking J, Metcalf BS, Jeffery AN, Streeter AJ, Voss LD, Wilkin TJ. Divergence between HbA1c and fasting glucose through childhood: implications for diagnosis of impaired fasting glucose (Early Bird 52). Pediatr Diabetes. 2014; 15:214–219. PMID: 25705748.
34. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care. 2008; 31:1991–1996. PMID: 18628569.
35. Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Discordance between fasting glucose-based and hemoglobin A1c-based diagnosis of diabetes mellitus in Koreans. Diabetes Res Clin Pract. 2011; 91:e8–e10. PMID: 20970868.

36. Li J, Ma H, Na L, Jiang S, Lv L, Li G, Zhang W, Na G, Li Y, Sun C. Increased hemoglobin A1c threshold for prediabetes remarkably improving the agreement between A1c and oral glucose tolerance test criteria in obese population. J Clin Endocrinol Metab. 2015; 100:1997–2005. PMID: 25751104.

37. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007; 30:2453–2457. PMID: 17536077.

38. Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, Narayan KM, Koch DD, Phillips LS. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010; 152:770–777. PMID: 20547905.
39. Cohen RM, LeCaire TJ, Lindsell CJ, Smith EP, D'Alessio DJ. Relationship of prospective GHb to glycated serum proteins in incident diabetic retinopathy: implications of the glycation gap for mechanism of risk prediction. Diabetes Care. 2008; 31:151–153. PMID: 17909088.
Fig. 1
Flow chart for the selection of study participants. KNHANES, Korea National Health and Nutrition Examination Survey; HbA1c, glycosylated hemoglobin.
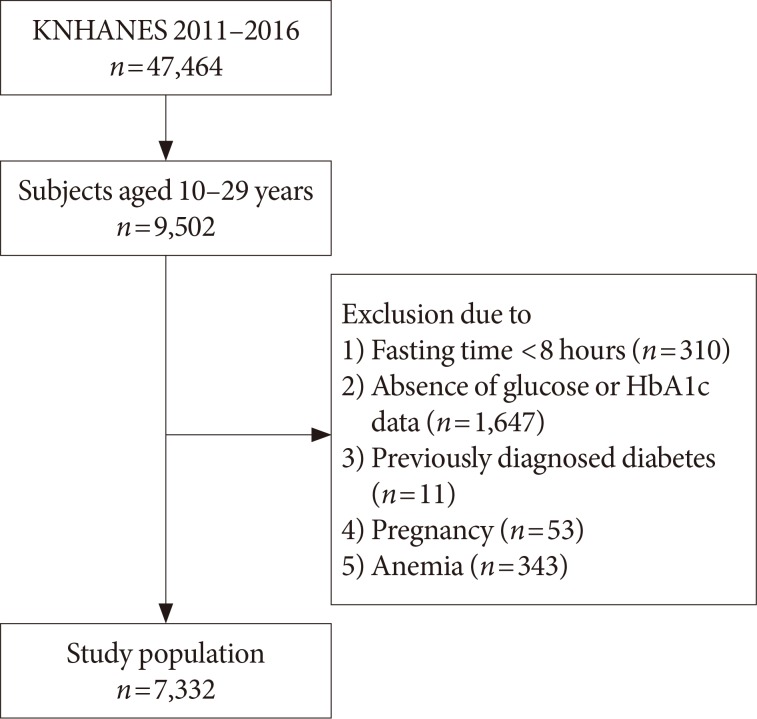
Fig. 2
Receiver operator characteristic curves for detection of (A) impaired fasting glucose (fasting plasma glucose ≥100 mg/dL) and (B) diabetes mellitus (fasting plasma glucose ≥126 mg/dL) according to glycosylated hemoglobin levels. (A) The area under the curve (AUC) (95% confidence interval [CI]) for impaired fasting glucose was 0.649 (95% CI, 0.648 to 0.650) in the youth group (10 to 19 years of age) and 0.700 (95% CI, 0.699 to 0.701) in the young adult group (20 to 29 years of age) (P<0.001). (B) The AUC (95% CI) for diabetes mellitus was 0.996 (95% CI, 0.996 to 0.996) in the youth group and 0.962 (95% CI, 0.962 to 0.963) in the young adult group (P<0.001).
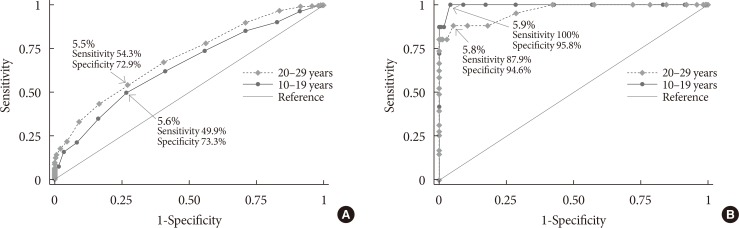
Table 1
Fasting plasma glucose level according to HbA1c category and age group
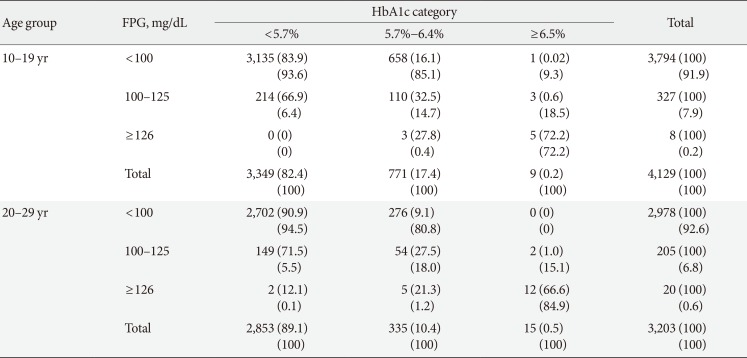
Table 2
Diagnostic performance of HbA1c according to cutoff values by the present study and ADA/KDA
Table 3
Cutoff values of glycosylated hemoglobin for prediabetes and diabetes
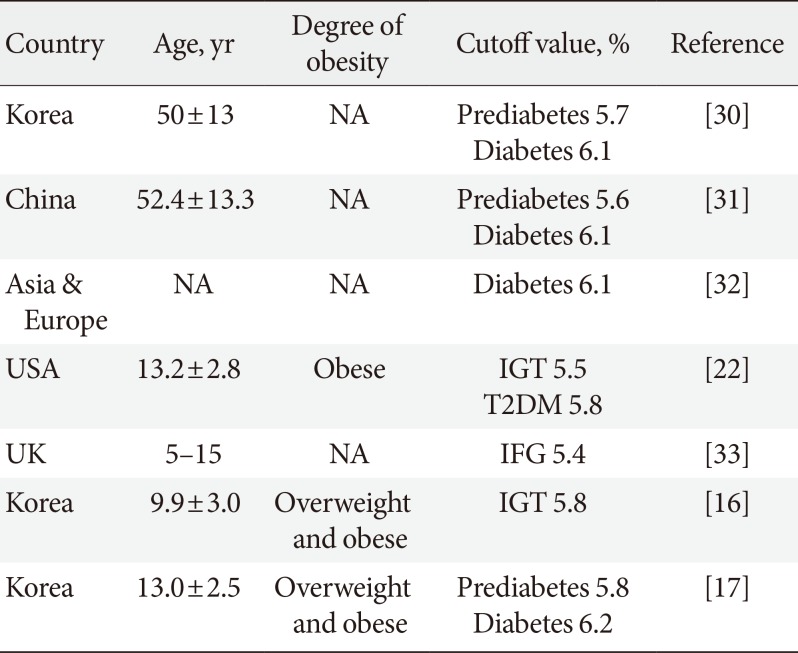
| Country | Age, yr | Degree of obesity | Cutoff value, % | Reference |
|---|---|---|---|---|
| Korea | 50±13 | NA | Prediabetes 5.7 | [30] |
| Diabetes 6.1 | ||||
| China | 52.4±13.3 | NA | Prediabetes 5.6 | [31] |
| Diabetes 6.1 | ||||
| Asia & Europe | NA | NA | Diabetes 6.1 | [32] |
| USA | 13.2±2.8 | Obese | IGT 5.5 | [22] |
| T2DM 5.8 | ||||
| UK | 5–15 | NA | IFG 5.4 | [33] |
| Korea | 9.9±3.0 | Overweight and obese | IGT 5.8 | [16] |
| Korea | 13.0±2.5 | Overweight and obese | Prediabetes 5.8 | [17] |
| Diabetes 6.2 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download