Abstract
Background
Methods
Results
ACKNOWLEDGMENTS
References
SUPPLEMENTARY MATERIAL
Supplementary Fig. 1
Table 1
Baseline characteristics according to four groups categorized by sex-specific medians of baseline serum albumin and uric acid
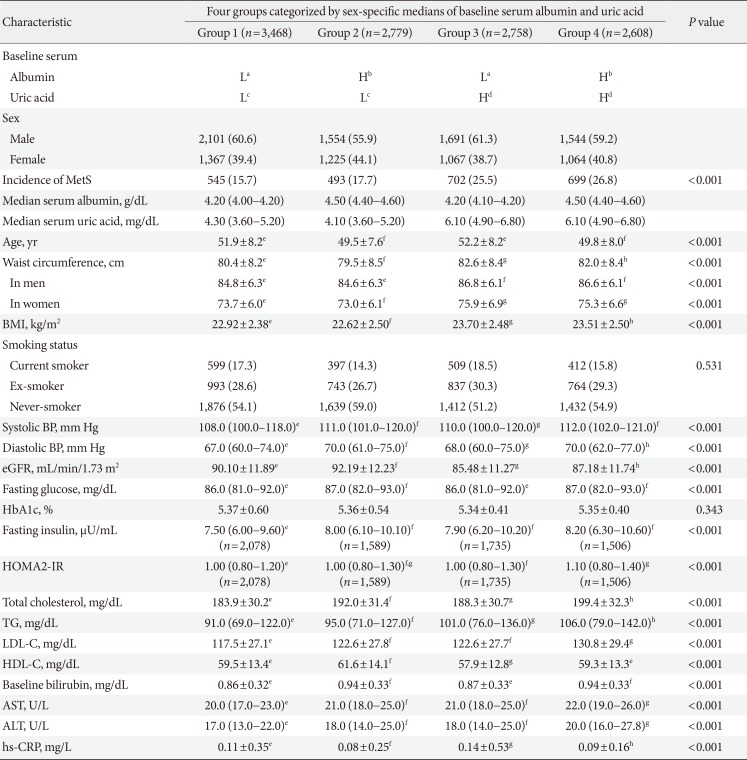
Values are presented as number (%), median (interquartile range), or mean±standard deviation.
L, lower; H, higher; MetS, metabolic syndrome; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HOMA2-IR, homeostatic model assessment index 2 for insulin resistance; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; hs-CRP, high-sensitivity C-reactive protein.
Baseline serum albumin aL (3.1 to 4.3 g/dL in males, 3.2 to 4.2 g/dL in females), bH (4.4 to 5.2 g/dL in males, 4.3 to 5.1 g/dL in females); Baseline serum uric acid cL (0.7 to 5.8 mg/dL in males, 0.5 to 4.1 mg/dL in females), dH (5.9 to 12.1 mg/dL in males, 4.2 to 7.7 mg/dL in females), e,f,g,hDifferent superscript letters imply significantly different values by the post hoc analysis (Tukey's multiple comparison test following a one-way analysis of variance or Mann-Whitney U test using Bonferroni correction following the Kruskal-Wallis test).
Table 2
Correlations between baseline serum albumin, serum uric acid and other parameters
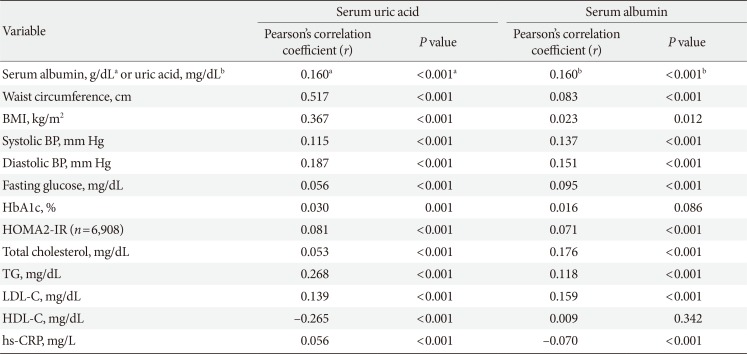
BMI, body mass index; BP, blood pressure; HbA1c, glycosylated hemoglobin; HOMA2-IR, homeostatic model assessment index 2 for insulin resistance; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
aSerum albumin (g/dL), bSerum uric acid (mg/dL).
Table 3
HRs and 95% CIs for incident metabolic syndrome according to four groups categorized by sex-specific medians of baseline serum albumin and uric acid
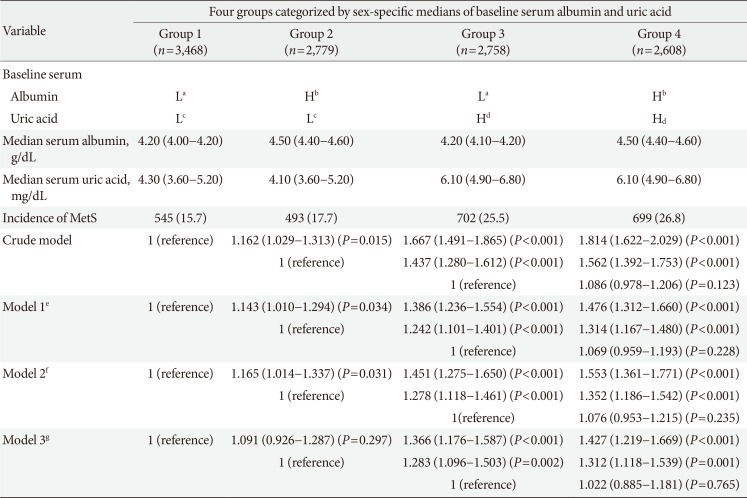
Values are presented as HR (95% CI).
HR, hazard ratio; CI, confidence interval; L, lower; H, higher; MetS, metabolic syndrome.
Baseline serum albumin aL (3.1 to 4.3 g/dL in males, 3.2 to 4.2 g/dL in females), bH (4.4 to 5.2 g/dL in males, 4.3 to 5.1 g/dL in females); Baseline serum uric acid cL (0.7 to 5.8 mg/dL in males, 0.5 to 4.1 mg/dL in females), dH (5.9 to 12.1 mg/dL in males, 4.2 to 7.7 mg/dL in females), eAdjusted for age, body mass index, waist circumference, systolic blood pressure, alanine aminotransferase, estimated glomerular filtration rate, fasting glucose, smoking status, medication (statin and aspirin), baseline diabetes mellitus prevalence, low density lipoprotein cholesterol, and high-sensitivity C-reactive protein, fAdjusted for Model 1+alcohol history (n=9,483), gAdjusted for Model 1+homeostasis model assessment index 2 for insulin resistance (n=6,908).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download