1. Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013; 169:166–176. PMID:
24063931.

2. van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, Blaak EE, Diamant M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011; 96:459–467. PMID:
21084401.

3. Pezzilli R, Calculli L. Pancreatic steatosis: is it related to either obesity or diabetes mellitus? World J Diabetes. 2014; 5:415–419. PMID:
25126389.

4. Szczepaniak LS, Victor RG, Mathur R, Nelson MD, Szczepaniak EW, Tyer N, Chen I, Unger RH, Bergman RN, Lingvay I. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012; 35:2377–2383. PMID:
22968187.
5. Heni M, Machann J, Staiger H, Schwenzer NF, Peter A, Schick F, Claussen CD, Stefan N, Haring HU, Fritsche A. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010; 26:200–205. PMID:
20225188.

6. Begovatz P, Koliaki C, Weber K, Strassburger K, Nowotny B, Nowotny P, Mussig K, Bunke J, Pacini G, Szendrodi J, Roden M. Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia. 2015; 58:1646–1655. PMID:
25740696.

7. Kim MK, Chun HJ, Park JH, Yeo DM, Baek KH, Song KH, Chung DJ, Kwon HS. The association between ectopic fat in the pancreas and subclinical atherosclerosis in type 2 diabetes. Diabetes Res Clin Pract. 2014; 106:590–596. PMID:
25444353.

8. Son JW, Jang EH, Kim MK, Kim IT, Roh YJ, Baek KH, Song KH, Yoon KH, Cha BY, Lee KW, Son HY, Kwon HS. Diabetic retinopathy is associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011; 91:253–259. PMID:
21129801.

9. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL. The task force for the management of arterial hypertension of the European Society of Hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007; 28:1462–1536. PMID:
17562668.
10. Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012; 107:1852–1858. PMID:
23032980.

11. Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015; 38:150–158. PMID:
25538311.

12. Catanzaro R, Cuffari B, Italia A, Marotta F. Exploring the metabolic syndrome: nonalcoholic fatty pancreas disease. World J Gastroenterol. 2016; 22:7660–7675. PMID:
27678349.

13. Matsumoto S, Mori H, Miyake H, Takaki H, Maeda T, Yamada Y, Oga M. Uneven fatty replacement of the pancreas: evaluation with CT. Radiology. 1995; 194:453–458. PMID:
7824726.

14. Itai Y, Saida Y, Kurosaki Y, Kurosaki A, Fujimoto T. Focal fatty masses of the pancreas. Acta Radiol. 1995; 36:178–181. PMID:
7710800.

15. Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011; 8:169–177. PMID:
21304475.

16. Yokota K, Fukushima M, Takahashi Y, Igaki N, Seino S. Insulin secretion and computed tomography values of the pancreas in the early stage of the development of diabetes. J Diabetes Investig. 2012; 3:371–376.

17. Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, Kim YH, Kim JH, Yoon YS, Han HS, Kang HS. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology. 2014; 271:104–112. PMID:
24475851.

18. Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY, Son BK, Kim SH, Jo YJ, Park YS, Kim YS. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009; 15:1869–1875. PMID:
19370785.

19. Pacifico L, Di Martino M, Anania C, Andreoli GM, Bezzi M, Catalano C, Chiesa C. Pancreatic fat and β-cell function in overweight/obese children with nonalcoholic fatty liver disease. World J Gastroenterol. 2015; 21:4688–4695. PMID:
25914480.

20. Lingvay I, Esser V, Legendre JL, Price AL, Wertz KM, Adams-Huet B, Zhang S, Unger RH, Szczepaniak LS. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009; 94:4070–4076. PMID:
19773401.

21. Gaborit B, Abdesselam I, Kober F, Jacquier A, Ronsin O, Emungania O, Lesavre N, Alessi MC, Martin JC, Bernard M, Dutour A. Ectopic fat storage in the pancreas using 1H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes (Lond). 2015; 39:480–487. PMID:
25042860.

22. Lim S, Bae JH, Chun EJ, Kim H, Kim SY, Kim KM, Choi SH, Park KS, Florez JC, Jang HC. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 2014; 51:739–748. PMID:
24671510.

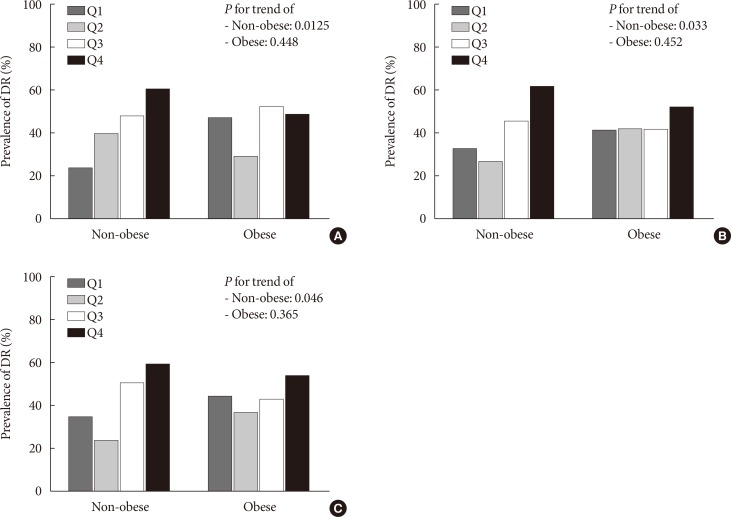
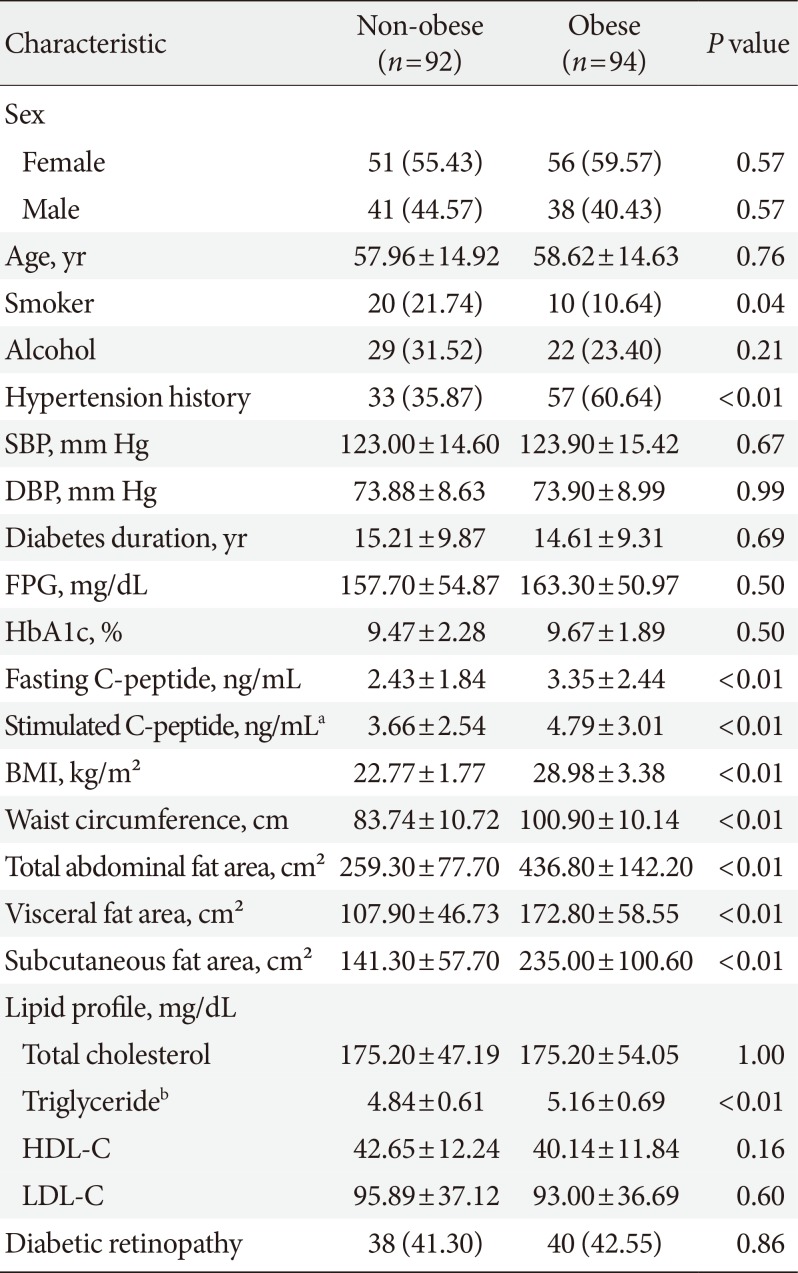
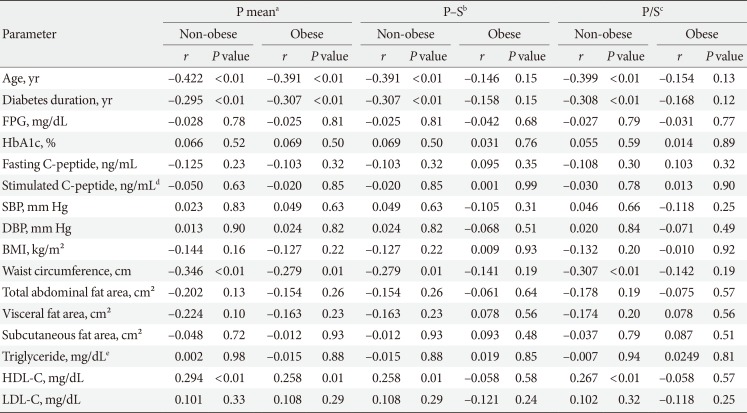
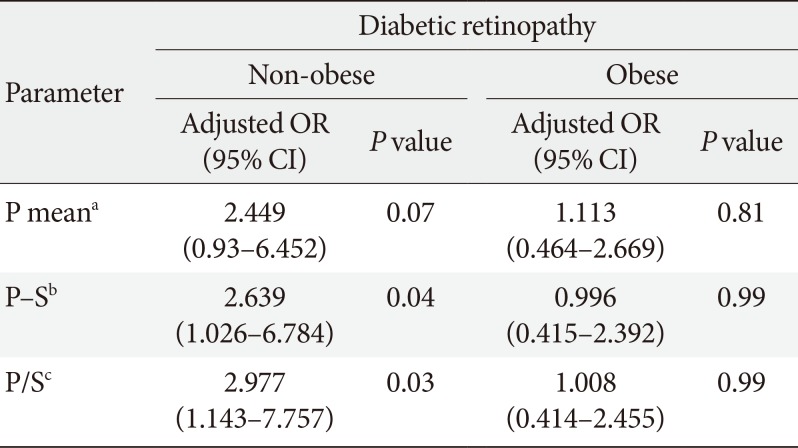




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download