Abstract
Brown fat is a specialized fat depot that can increase energy expenditure and produce heat. After the recent discovery of the presence of active brown fat in human adults and novel transcription factors controlling brown adipocyte differentiation, the field of the study of brown fat has gained great interest and is rapidly growing. Brown fat expansion and/or activation results in increased energy expenditure and a negative energy balance in mice and limits weight gain. Brown fat is also able to utilize blood glucose and lipid and results in improved glucose metabolism and blood lipid independent of weight loss. Prolonged cold exposure and beta adrenergic agonists can induce browning of white adipose tissue. The inducible brown adipocyte, beige adipocyte evolving by thermogenic activation of white adipose tissue have different origin and molecular signature from classical brown adipocytes but share the characteristics of high mitochondria content, UCP1 expression and thermogenic capacity when activated. Increasing browning may also be an efficient way to increase whole brown fat activity. Recent human studies have shown possibilities that findings in mice can be reproduced in human, making brown fat a good candidate organ to treat obesity and its related disorders.
Obesity has increased at an alarming rate worldwide. Since obesity is associated with many diseases, such as type 2 diabetes, insulin resistance, dyslipidemia, hypertension, cardiovascular disease, sleep apnea, and some types of cancer, the rapid increase in obesity is a major health burden [1]. Although there is an epidemic of obesity worldwide the effort to decrease this epidemic has not been effective and there is a growing need of novel therapeutics to prevent and treat obesity [2]. Obesity is thought to be caused by an imbalance between energy intake and energy consumption. Pharmacologic agents currently approved have the effect of mainly decreasing energy intake and there are currently no approved drug that can increase energy consumption.
Fat tissue had been considered a simple storage organ until the late 1980s. Many lines of evidence subsequently established adipose tissue as an endocrine organ secreting many hormones and cytokines that can influence systemic metabolism [3]. Moreover, recent work has established distinct adipose depots that play specific roles. In contrast to white fat, which mainly stores energy and is increased in obesity, brown fat generates heat (thermogenesis), thus increasing consumption of energy; as such, brown fat and the induction of brown fat-like properties in white fat, has been considered as a target for fighting obesity. Brown fat, also known as brown adipose tissue (BAT) by dissipating energy as heat, has an essential role of maintaining body temperature in rodents and human neonates [4]. In human adults, the existence of BAT was debated, and if present, thought to be a remnant, physiologically non-significant organ until recently. Several groups have shown using fluorodeoxy glucose (FDG)-positron emission tomography (PET) in combination with computed tomography (CT), the presence of brown fat and the association with age, leanness, and environmental temperature with brown fat activity [567]. These studies have made brown fat to be a plausible target organ for the management of obesity and its related diseases in human. By increasing the amount and/or activity of brown fat, energy consumption can be increased, and this will lead to a negative energy balance. The field of basic science has also shown much progress in the understanding of brown fat development and physiology [48]. The finding of an inducible thermogenic adipocyte, the beige (inducible, brite) adipocyte has also been studied extensively.
In this review, we will review the development and function of brown and beige adipocytes, the role and significance of brown fat and browning in the treatment of obesity and diabetes, and the relevance in humans.
Brown adipocytes are the major constituents of BAT, occupying the largest area. Traditionally adipocytes have been divided into white adipocytes and brown adipocytes. White adipocytes and brown adipocytes both have fat droplets but these differ in morphology and function. White adipocytes have a unilocular fat droplet, relatively few mitochondria, and mainly store fat. In contrast, brown adipocytes have multilocular fat droplets, a high content of mitochondria, and a high level of expression of uncoupling protein 1 (UCP1) in the inner membrane of mitochondria. UCP1 is a critical player in allowing electrons to be released rather than stored, resulting in heat release. The most important and well-studied factor influencing brown adipocytes is norepinephrine (NE). NE is able to affect activation, cell proliferation and differentiation of brown adipocytes. NE mainly functions through the β-adrenergic receptor, especially the β3-adrenoreceptor. After receptor binding, via adenylyl cyclase activation, cyclic adenosine monophosphate (cAMP) level is increased and protein kinase A (PKA) is activated leading to triglyceride breakdown and release of free fatty acids (FFAs) which are acute substrates for thermogenesis and regulators of the activity of UCP1. PKA can phosphorylate cAMP-response-element-binding (CREB) and p38 mitogen-activated protein kinases (MAPK) which drives a thermogenic transcriptional response including the induction of UCP1 gene expression [4910]. Brown adipocytes can dissipate chemical energy stored as triglycerides by channeling fatty acids into β oxidation when activated. UCP1 is able to uncouple electron transport from adenosine triphosphate (ATP) production and produces heat in brown adipocytes [811].
Beige adipocytes (also called 'brite,' 'brown-like,' 'inducible brown') are an inducible form of thermogenic adipocytes and have a low UCP1 content in basal conditions but when induced have a high UCP1 expression level and increased energy consumption [412]. The origin and characteristics of classical brown adipocytes and beige adipocytes are different (Table 1). Classical brown adipocytes originate from precursor cells in the embryonic mesoderm that express Myf5 and Pax7 [13]. Beige adipocytes are thought to come from a distinct cell lineage mainly of a Myf5 negative lineage. The origin of beige adipocytes residing in white fat tissue has been a focus of great debate [4]. Some studies have shown that the origin was trans-differentiation from mature white adipocytes [1415] whereas a recent study using a pulse-chase fate-mapping technique, has shown that most of the beige adipocytes arise by de novo differentiation from a precursor population [16]. Beige and white adipocytes arising from the white adipose tissue (WAT) are thought to arise from a different type of precursor cells. Wu et al. [17] has identified two types of preadipocytes (white and beige) using global gene profiling and differentiation analysis. Beige preadipocytes expressed Cd137 and transmembrane protein 26 (Tmem26) on the cell surface and only the adipocytes differentiated from the beige preadipocytes were able to induce a thermogenic gene program with β-adrenergic agonist treatment [17]. Beige adipocytes are most abundant in the subcutaneous depots of rodents [14]. In epididymal WAT of male mice which is a visceral fat depot, beige adipocytes develop from a bipotent precursor that can also differentiate into white adipocytes. These bipotent precursors express platelet-derived growth factor receptor α and represent a subpopulation of stromal cells expressing the common stem cell markers CD34 and Sca-1 [18].
Master regulators of adipogenesis, peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding proteins (C/EBPs), are involved in the differentiation of both white and brown adipocytes [8]. A large zinc finger-containing transcriptional factor, PR domain containing 16 (PRDM 16) plays a major role in the cell fate decision process promoting the development of brown adipocytes from Myf5 expressing precursors [13]. PRDM16 was also able to induce brown fat cells in WAT [19]. PRDM16 stimulates brown adipogenesis by binding to PPARγ coactivator 1α (PGC-1α), PGC-1β, C/EBP-β and PPARγ and activating its transcriptional function [131920].
BAT, due to its ability to uncouple electron gradients, generate ATP and promote energy dissipation, can be a target for obesity management. In rodents, BAT is found in the interscapular, perirenal, and periaortic regions. In human infants, similar to rodents, BAT depots mainly reside in the interscapular and perirenal area and this depot gradually disappears with increasing age [2122]. Human adults mainly have BAT in the cervical, supraclavicular, axillary and paravertebral areas as demonstrated in studies using 18F-FDG-PET/CT [567]. BAT is composed of brown adipocytes with a high content of the BAT specific mitochondrial protein, UCP1 [23]. UCP1 has no activity in basal states but can be activated by long chain fatty acids generated within the mitochondrial inner membrane [24]. BAT is highly vascularized and densely innervated by the sympathetic nervous system. BAT is activated by cold exposure and is mainly controlled by the sympathetic nervous system. Sympathetic activation of BAT results in increased thermogenic gene expression and recruitment of enzymes needed for uptake, mobilization and oxidation of lipids [23]. The thyroid hormone system also plays a synergistic role and is essential for BAT activation [25]. BAT can also be activated by increasing caloric intake in rodents and this process is termed diet-induced thermogenesis [1126]. This will be of great relevance to the BAT based obesity treatment but this has not yet been confirmed in humans.
Clusters of thermogenic beige adipocytes can be seen in traditional WAT depots after chronic cold exposure or through the genetic manipulation of specific pathways [27]. Whether white adipocytes can be induced to transdifferentiate into a more brown adipocyte like state remains unresolved. Regardless, both processes are referred to in general as 'browning' of WAT [8]. Chronic treatment with β3-adrenergic receptor activators and thiazolidinediones can also induce the browning process [2829]. Increasing the amount of beige fat may be a valid method to increase the amount of BAT and increase energy consumption. Accordingly, many rodent models have shown that increased browning of the WAT was able to resist diet induced obesity and improve metabolism [430313233]. Recent studies showing that the adult BAT shows characteristics of beige adipocytes reinforces the browning of adipose tissue as an interesting therapeutic possibility [17]. The browning process is regulated by a complex hormonal interplay and many environmental factors such as chronic cold exposure, exercise and environmental enrichment (Table 1) [834].
Cold is sensed by thermoreceptors and leads to a sympathetic outflow to BAT through a neural circuitry [35]. Classically, NE released from the sympathetic nerve terminal activates the BAT thermogenic program via PKA and p38 MAPK signaling and leads to lipolysis and production of FFA for UCP1 activation [34]. Recent studies have elucidated an efferent beige fat thermogenic circuit-consisting of eosinophils, type 2 cytokines interleukin (IL)-4/13, and alternatively activated macrophages-which regulate the development of cold-induced beige fat. Cold exposure induces eosinophil IL-4/13 production and this leads to M2 macrophage activation which induces tyrosine hydroxylase expression and catecholamine production leading to browning of subcutaneous WAT [3637]. Meteorin-like (Metrnl) is a PGC-1α4-regulated hormone that is induced upon exercise and cold exposure promoting browning by inducing IL-4/13 and M2 macrophage activation, which is required for long-term adaptations to cold temperatures [37].
Exercise has been reported to increase browning of WAT and increase energy expenditure. There is a possibility that this mechanism may be an additional pathway leading to the chronic benefits of exercise training on glucose and lipid metabolism [38]. PGC-1α is induced in the muscle by exercise and stimulates many beneficial effects of exercise in muscle, including mitochondrial biogenesis and muscle fibre-type switching [39]. PGC-1α is able to increase expression of FNDC5 (fibronectin domain-containing 5), a membrane protein that is cleaved and secreted as irisin. Although controversial, irisin has been reported to be induced by exercise in mice and humans and capable of inducing browning of WAT, resulting in improvements in obesity and glucose homeostasis [38]. Metrnl is a myokine induced by resistance exercise and PGC-1α4. Metrnl is able to promote activation of M2 macrophages and catecholamine production from these cells to induce WAT browning [37]. IL-6 is a major myokine induced by exercise and has been reported to play a role in exercise induced WAT browning [40].
Living in an enriched environment with complex physical and social stimulation was able to induce browning of WAT in mice. This is mediated by induction of hypothalamic brain-derived neurotropic factor (BDNF) expression, which leads to selective sympathetic nervous system activation of white fat to induce browning and increase energy dissipation. Environmental enrichment was able to resist diet-induced obesity through the browning effect. Hypothalamic overexpression of BDNF was able to reproduce the enrichment-associated activation of browning of WAT and the associated lean phenotype [41].
There are many novel factors and signaling pathways that can induce brown and/or beige. Among these factors fibroblast growth factors 21 (FGF21) [42434445], cardiac natriuretic peptides (NPs) [46], retinaldehyde (Rald) [31], bone morphongenetic proteins (BMPs) [4748] are secreted molecules that can activate BAT and/or induce browning in WAT. FGF21 is an endocrine factor produced in liver, BAT, and skeletal muscle [49]. Cold exposure induces FGF21 gene transcription and FGF21 release in brown adipocytes via cAMP-mediated PKA and p38 MAPK activation [43]. Cold induced increase in plasma FGF21 levels was also observed in humans [50]. FGF21 plays a physiological role in browning of WAT. Adipose-derived FGF21 acts in an autocrine and paracrine manner to increase UCP1 and other thermogenic genes in fat tissues. These processes are in part regulated by enhancing adipose tissue PGC-1α protein levels without affecting mRNA expression. Mice deficient of FGF21 showed an impaired ability to adapt to chronic cold exposure and decreased browning of WAT [45].
The cardiac NPs, atrial NP (ANP) and its ventricular companion, brain-type NP (BNP) are key hormones in fluid and hemodynamic homeostasis. NPs are expressed in adipose tissue and ANP was shown to increase lipolysis in human adipocytes [51]. Bordicchia et al. [46] showed that NPs promote browning of white adipocytes to increase energy expenditure and increase thermogenic gene expression in BAT. In human adipocytes, ANP and BNP activated PGC-1α and UCP1 expression, induced mitochondriogenesis and increased uncoupled and total respiration. ANP and β-adrenergic receptor agonists additively enhanced expression of brown fat and mitochondrial markers in a p38 MAPK-dependent manner. Mice exposed to cold temperatures had increased levels of circulating NPs and a higher expression of NP signaling receptor in BAT and WAT. Infusion of BNP into mice robustly increased Ucp1 and Pgc-1α expression in WAT and BAT with corresponding elevation of respiration and energy expenditure. NP bind to NP receptor A, whose intracellular domain possesses guanylyl cyclase activity to generate cyclic guanosine monophosphate (cGMP). Activation of guanylyl cyclase and generation of cGMP leads to activation of cGMP-dependent protein kinase (PKG) [46]. PKG works in parallel with the β-adrenergic-PKA pathway to trigger lipolysis and stimulate thermogenesis [4].
BMPs are members of the transforming growth factor β superfamily of extracellular signaling proteins that influence the differentiation of mesenchymal stem cells. Several BMP members have been shown to play a role in the regulation of adipocyte differentiation and energy expenditure [52]. BMP7 promotes differentiation of brown preadipocytes through induction of PRDM16 and PGC-1α, which are early regulators of brown fat fate. BMP7 can also trigger commitment of mesenchymal progenitor cells to a brown adipocyte lineage. BMP7 knockout embryos show a marked decrease in brown fat and almost complete absence of UCP1. Adenoviral expression of BMP7 in mice results in a significant increase in BAT mass and leads to an increase in energy expenditure and a reduction in weight gain [53]. BMP7 is also able to induce differentiation of Sca-1 positive progenitor cells from muscle and subcutaneous WAT into brown-like adipocytes [47]. BMP4 is expressed at an elevated level in WAT of lean human subjects. Induced expression of BMP4 in WAT induces browning of WAT and increases whole body metabolic rate and insulin sensitivity. PGC-1α mediates the BMP4 induced browning of WAT [48]. BMP8B appears to have both central and peripheral actions. BMP8B is induced by nutritional and thermogenic factors in mature BAT, increasing the response to noradrenaline through enhanced p38MAPK/CREB signaling and increased lipase activity. Central BMP8B treatment increases sympathetic activation of BAT, dependent on the level of AMP-activated protein kinase (AMPK) in the ventromedial nucleus of the hypothalamus. BMP8B null mice show decreased energy expenditure and increased body weight gain. BMP8B is a thermogenic protein that can act centrally with hypothalamic AMPK to regulate energy expenditure [54].
Retinaldehyde dehydrogenase 1 (Aldh1a1) is the rate-limiting enzyme that converts Rald to retinoic acid. Aldh1a1 is expressed predominately in WAT, with the highest levels found in visceral WAT in mice and humans. Deficiency of the Aldh1a1 in mice induced browning of WAT that drove uncoupled respiration and adaptive thermogenesis, protecting against diet-induced obesity and diabetes [31]. In addition to this phenotype in the knockout mouse, antisense oligonucleotide-mediated Aldh1a1 knockdown in visceral WAT was able to replicate this response, promoting browning of WAT with increased thermogenesis, decreased weight gain and improved glucose homeostasis in high fat diet (HFD) fed mice. These effects seem to be related to increased Rald levels, since Rald was able to induce UCP1 mRNA and protein levels in white adipocytes by selectively activating the retinoic acid receptor, recruiting PGC-1α and inducing UCP1 promoter activity [31].
Increasing BAT amount and/or increasing BAT activity can lead to increased energy expenditure which will be favorable in preventing and managing obesity. Increasing BAT amount and/or activity will also have a direct beneficial effect on glucose and lipid metabolism independent from the effect on body weight (Fig. 1) [55]. Cold exposure can accelerate plasma clearance of triglycerides as a result of increased uptake into BAT in mice. This process is dependent on local lipoprotein lipase activity and transmembrane receptor CD36. In pathophysiological settings of dyslipidemia and insulin resistance, cold exposure corrected hyperlipidemia and improved the deleterious effects of insulin resistance [55]. BAT can clear a substantial portion of total glucose and triglycerides from the circulation when activated. Therefore targeting BAT will have beneficial effects on metabolic disorders related to obesity.
Evidence of the beneficial effects of increasing brown fat amount can be seen from genetic models of increased BAT [4] and BAT transplantation [56]. BAT transplantation into the visceral cavity in mice resulted in improved glucose tolerance, increased insulin sensitivity, lower body weight, and decreased fat mass. BAT transplantation also resulted in complete reversal of HFD-induced insulin resistance [56]. The beneficial effect of BAT transplantation can also be attributed to secreted factors from the BAT. Another method to increase BAT amount can be increased browning of WAT.
PGC-1α plays a central role in thermogenic activation of adipocytes. PGC-1α was discovered as a cold-induced interacting partner of PPARγ in brown fat [57]. PGC-1α is a master regulator of mitochondrial biogenesis and oxidative metabolism and in adipocytes, can also induce the expression of UCP1 and other thermogenic components [4]. PGC-1α is essential for cold induced and β-adrenergic agonist induced thermogenic activation of brown adipocytes [58]. PGC-1α expression and activity are regulated directly by the β-adrenergic pathway, linking physiologic activator of brown fat thermogenesis and the transcriptional machinery in brown adipocytes [459]. cAMP and PKA dependent activation of p38 MAPK in brown adipocytes phosphorylates activating transcription factor 2 (ATF-2) and PGC-1α. Activation of ATF-2 by p38 MAPK additionally serves as the cAMP sensor that increases expression of the PGC-1α gene itself in BAT [59].
The beneficial effects of BAT have been shown mainly in animal models, leaving unresolved whether thermogenesis might be harnessed in humans. However, the field of the study of human brown fat is emerging rapidly and during recent years, many researchers using sophisticated methods are tackling the issue of the characteristics of human brown fat and its therapeutic possibilities [3460]. Many studies have tried to characterize the human BAT using markers of classical brown adipocytes and beige adipocytes of mice. The interscapular BAT that is seen in human infants show characteristics of classic brown adipocytes [22]. The supraclavicular BAT in adult humans express high levels of beige-adipocyte enriched genes, including T-box transcription factor 1 (Tbx1), Tmem26, Cd137 [17]. On the contrary recent studies have shown that adult BAT express classical brown adipocyte-selective markers, including upregulation of miR260, miR-133b, LIM homeobox protein 8 (Lhx8) and zinc finger protein 1 (Zic1) and down regulation of homeobox C8 (Hoxc8) and homeobox C9 (Hoxc9) [61]. This discrepancy is probably due to the heterogeneity of human BAT and the difference in gene signature according to depth, with deeper depots showing more characteristics of classical brown [62]. Shinoda et al. [63] isolated clonally derived adipocytes from stromal vascular fractions of adult human BAT and globally analyzed their molecular signature and found it resembled those of murine beige adipocytes. Sidossis et al. [64] showed that human subcutaneous WAT undergoes browning and is associated with increased whole-body metabolic rate using burn trauma as a model of prolonged adrenergic stress.
There have been many clinical studies in human adults that suggest the beneficial effect of activating BAT [34]. Ouellet et al. [65] quantified BAT oxidative metabolism and glucose and nonesterified fatty acid (NEFA) turnover in healthy men under controlled cold exposure conditions. Upon cold exposure, there was increased NEFA and glucose uptake and activation of oxidative metabolism in BAT associated with a 1.8-fold increase in whole-body energy expenditure [65]. Yoneshiro et al. [66] showed that daily 2 hours cold exposure at 17℃ for 6 weeks resulted in increases in BAT activity and cold induced increments of energy expenditure and a concomitant decrease in body fat mass. Chondronikola et al. [67] reported that prolonged cold exposure for 5 to 8 hours was able to increase resting energy expenditure (REE) by 15% in BAT positive men, and plasma glucose (30%) and FFA (70%) contributed to the observed increase in REE. Glucose disposal was increased in BAT and whole-body glucose disposal was significantly increased [67]. In type 2 diabetes subjects, 10 days of cold acclimation increased peripheral insulin sensitivity by 43%. Basal skeletal muscle glucose transporter type 4 (GLUT4) translocation was markedly increased and glucose uptake in skeletal muscle was increased after cold acclimation [68]. In summary, BAT activation through cold exposure was able to increase energy expenditure and have beneficial effects on glucose metabolism supporting its role in treating obesity and related metabolic disorders in humans.
The current epidemic of obesity and its related complications like diabetes and heart disease is alarming and underscores the need for novel therapeutics. Targeting BAT and browning of WAT has arisen as a potentially important, efficient strategy to treat obesity and its related metabolic disorders. Increasing the amount and/or activity of theromogenesis will lead to a negative energy balance by increasing energy expenditure; and decrease blood glucose and triglyceride substantially, improving diabetes and dyslipidemia. Many recent human studies have shown that the effects of manipulating brown fat pathways seen in mice are also present in humans. However, more studies are needed to understand the differential physiology and role of brown and beige adipocyte. Understanding their specific mechanism and physiology will enable the development of more safe and efficient therapeutic agents for the treatment of obesity and related metabolic disorders.
Figures and Tables
Fig. 1
Brown adipose tissue (BAT) targeted treatment of obesity and related metabolic disorders. Increasing BAT amount and/or BAT activity leads to increased activation of uncoupling protein 1 (UCP1) and thermogenesis leading to energy expenditure which will have anti-obesity effects. On the other hand, BAT can also increase the uptake of blood glucose and lipid. Glucose and fatty acids are oxidized or stored as glycogen or triglyceride. This results in decrease in blood glucose and lipid and increase in insulin sensitivity, leading to anti-diabetic and anti-dyslipidemic effects.
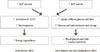
Table 1
Characteristics of brown and beige adipocytes

Lhx8, LIM homeobox protein 8; Zic1, zinc finger protein 1; Tmem26, transmembrane protein 26; Tbx1, T-box transcription factor 1; FGF21, fibroblast growth factor 21; ANP, atrial natriuretic peptide; BNP, brain-type natriuretic peptide; BMP, bone morphogenetic protein; BDNF, brain-derived neurotropic factor.
ACKNOWLEDGMENTS
This work was supported by a research grant from the Korean Diabetes Association (2010).
References
1. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014; 129:25 Suppl 2. S102–S138.
2. Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD. Endocrine Society. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015; 100:342–362.
3. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004; 89:2548–2556.
4. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013; 19:1252–1263.
5. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009; 360:1509–1517.
6. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009; 360:1500–1508.
7. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009; 360:1518–1525.
8. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014; 10:24–36.
9. Cao W, Medvedev AV, Daniel KW, Collins S. Beta-adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem. 2001; 276:27077–27082.
10. Thonberg H, Fredriksson JM, Nedergaard J, Cannon B. A novel pathway for adrenergic stimulation of cAMP-response-element-binding protein (CREB) phosphorylation: mediation via alpha1-adrenoceptors and protein kinase C activation. Biochem J. 2002; 364(Pt 1):73–79.
11. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84:277–359.
12. Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions. Endocrinology. 2013; 154:2992–3000.
13. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008; 454:961–967.
14. Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012; 53:619–629.
15. Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000; 279:C670–C681.
16. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013; 19:1338–1344.
17. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012; 150:366–376.
18. Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012; 15:480–491.
19. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007; 6:38–54.
20. Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009; 460:1154–1158.
21. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972; 112(Pt 1):35–39.
22. Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerback S. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013; 19:631–634.
23. Townsend KL, Tseng YH. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab. 2014; 25:168–177.
24. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012; 151:400–413.
25. Whittle A, Relat-Pardo J, Vidal-Puig A. Pharmacological strategies for targeting BAT thermogenesis. Trends Pharmacol Sci. 2013; 34:347–355.
26. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009; 9:203–209.
27. Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984; 167:10–14.
28. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992; 103(Pt 4):931–942.
29. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010; 285:7153–7164.
30. Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010; 328:1158–1161.
31. Kiefer FW, Vernochet C, O'Brien P, Spoerl S, Brown JD, Nallamshetty S, Zeyda M, Stulnig TM, Cohen DE, Kahn CR, Plutzky J. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012; 18:918–925.
32. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011; 121:96–105.
33. Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ, Laznik D, Kamenecka TM, Song X, Liedtke W, Mootha VK, Puigserver P, Griffin PR, Clapham DE, Spiegelman BM. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012; 151:96–110.
34. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015; 125:478–486.
35. Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne). 2012; 3:pii00005.
36. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014; 157:1292–1308.
37. Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014; 157:1279–1291.
38. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–468.
39. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008; 454:463–469.
40. Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, Pilegaard H. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One. 2014; 9:e84910.
41. Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011; 14:324–338.
42. Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011; 17:736–740.
43. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011; 286:12983–12990.
44. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008; 149:6018–6027.
45. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012; 26:271–281.
46. Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012; 122:1022–1036.
47. Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011; 108:143–148.
48. Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, Liu Y, Sun X, Li YM, Jia WP, Tang QQ. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A. 2013; 110:E798–E807.
49. Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab. 2015; 26:231–237.
50. Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014; 19:302–309.
51. Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000; 14:1345–1351.
52. Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011; 32:387–403.
53. Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008; 454:1000–1004.
54. Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, Lopez M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012; 149:871–885.
55. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011; 17:200–205.
56. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013; 123:215–223.
57. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998; 92:829–839.
58. Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006; 3:333–341.
59. Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004; 24:3057–3067.
60. Celi FS, Le TN, Ni B. Physiology and relevance of human adaptive thermogenesis response. Trends Endocrinol Metab. 2015; 26:238–247.
61. Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, Pedersen BK, Moller K, Scheele C. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013; 17:798–805.
62. Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren PO, Mori MA, Molla M, Tseng YH. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013; 19:635–639.
63. Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, Nedergaard J, Sidossis LS, Kajimura S. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015; 21:389–394.
64. Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015; 22:219–227.
65. Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012; 122:545–552.
66. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013; 123:3404–3408.
67. Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014; 63:4089–4099.
68. Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jorgensen JA, Boekschoten MV, Hesselink MK, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbelt WD, Schrauwen P. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015; 21:863–865.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download