Abstract
The conventional glycemic indices used in management of diabetic patients includes A1c, fructosamine, 1,5-anhydroglucitol, and glycated albumin (GA). Among these indices, A1c is currently used as the gold standard. However, A1c cannot reflect the glycemic change over a relatively short period of time, and its accuracy is known to decrease when abnormalities in hemoglobin metabolism, such as anemia, coexist. When considering these weaknesses, there have been needs for finding a novel glycemic index for diagnosing and managing diabetes, as well as for predicting diabetic complications properly. Recently, several studies have suggested the potential of GA as an intermediate-term glycation index in covering the short-term effect of treatment. Furthermore, its role as a pathogenic protein affecting the worsening of diabetes and occurrence of diabetic complications is receiving attention as well. Therefore, in this article, we wanted to review the recent status of GA as a glycemic index and as a pathogenic protein.
The importance of accurately assessing glycemic status in the management of diabetic patients cannot be overemphasized. Various parameters including fasting glucose level, postprandial glucose level, and glycated hemoglobin are used to great effect in determining the glycemic status of patients, and their clinical significance has already been proven through numerous epidemiological and clinical studies. Although these three glycemic indices provide useful information, in several cases these indices alone are inadequate. For example, appropriately monitoring glycated hemoglobin level is difficult in the setting of an abnormal hemoglobin metabolic pathway, such as anemia, decreased renal function, and gestational diabetes. Furthermore, since glycated hemoglobin reflects a change in glycemic status over a period of 2 to 3 months, it is inappropriate in monitoring the therapeutic effects in earlier stages in the management of diabetes. Such limitations act as obstacles in the use of glycated hemoglobin levels in clinical settings.
Recently, the value of glycated albumin (GA) as a glycemic control marker has been validated in several studies. The half-life of albumin is approximately 15 days, and GA level is believed to reflect the glycemic change over a 2-week period. Therefore, GA can be useful in evaluating the therapeutic effect of recently substituted hypoglycemic agents at an early stage. GA can also act as a valuable glycemic control marker in diabetic patients with various comorbidities since it is unrelated to the metabolism of hemoglobin. This review attempts to cover various aspects of GA, from basic science to clinical usage, in detail, along with an overview of the recent studies on the subject.
Since the United Kingdom Prospective Diabetes Study and its follow-up studies, as well as the Diabetes Control and Complications Trial, little controversy remains that individualized intensive glucose control reduces or delays hyperglycemia-induced vascular complications in diabetic subjects [1-3]. To achieve and maintain intensive or acceptable glucose control, accurate and effective glycemic indicators, with which biological and clinical variability do not interfere, are essential. By overcoming many shortcomings of the methodology for markers of glucose control, glycemic indicators have contributed to reaching and maintaining glucose targets and thereby optimize glycemic control.
Glucose or diabetes monitoring parameters can be simply classified into indices of protein glycation or non-protein markers. Glycated protein indices including glycated hemoglobin (A1c), fructosamine (FA), and GA are currently the mainstay method for glycemic monitoring. Glycation of proteins within the body, such as plasma proteins, enzymes, hormones, matrix proteins, membrane, and intracellular proteins, etc., correlates with blood glucose levels and the duration of protein exposure to them. Thus, the relative amount of glycated protein serves as an indirect record of glycemic status over the period of protein turnover. Of the glucose monitoring markers using protein glycation, A1c, is considered the gold standard in the clinical setting. In addition, recent efforts addressing reference method standardization have empowered A1c as a diagnostic tool for diabetes [4]. However, the use of A1c as a glycation index faces some challenges that are not easily overcome. Because hemoglobin resides within erythrocytes, its glycation is affected by variant types of hemoglobin and a myriad of diseases influencing erythrocyte lifespan. Thus, A1c does not accurately represent glycemic control in such conditions. FA, named for its chemical similarity to fructose, represents the sum of all fructosamine residue (ketoamine) linkages resulting from glycated serum proteins. Thus, it is a measurement of glycated serum proteins, the most common of which is albumin [5]. Although FA is not influenced by diseases characterized by abnormal hemoglobin metabolism, it is strongly influenced by the concentration of serum proteins and low-molecular-weight substances coexisting in the plasma (e.g., bilirubin, hemoglobin, and uric acid) [6,7]. In order to overcome the drawbacks of FA while maintaining its advantages, the idea of using GA as a glycation index was developed. GA, which is theoretically not affected by disorders of hemoglobin metabolism or by the concentration of serum proteins and low-molecular-weight substances, is now gaining popularity as a useful glycation index for glucose monitoring. Because this method calculates the ratio of GA to total serum albumin, it is not influenced by the concentration of serum albumin [6]. Glycation of albumin involves a slow, non-enzymatic reaction initially involving the attachment of glucose or its derivatives to free amine groups of albumin, leading to formation of stable ketoamine [8]. Apolipoprotein B (Apo-B), a component of low density lipoproteins (LDLs), is also glycated; however, tests for glycated apo-B are unavailable in clinical practice. The particular interest in glycated LDL lies not in its use as a glycation index for glucose monitoring but in its involvement in the development of atherogenesis [9,10].
The currently available non-protein markers are 1,5-anhydroglucitol (1,5-AG) and glucose levels in blood or serum. The compound 1,5-AG is a circulating polyol molecule. Under normal physiological conditions, 1,5-AG filtered from the blood by kidney glomeruli is entirely reabsorbed by the renal tubules. Because of its structural similarity to glucose, 1,5-AG must compete with glucose for reabsorption, leading to a decline in plasma 1,5-AG levels when glucose in the circulation exceeds approximately 180 mg/dL [11]. Thus, 1,5-AG level is not actually a measure of mean glycose level but rather a measure of hyperglycemic excursions [5]. Though 1,5-AG reflects postprandial excursions more accurately than either A1c or FA [12], it is not recommended for glucose monitoring of patients whose renal hemodynamics are unstable. Another non-protein and non-glycation index is blood or serum glucose level, which could be checked and calculated by timed sampling. The glucose variation or fluctuation can be measured in the clinical setting using self-monitoring of blood glucose or a continuous glucose monitoring system. In addition, standard deviation, continuous overlapping net glycemic action, mean amplitude of glycemic excursion, and mean of the daily differences are also calculated for the assessment of glycemic variability for academic purpose [13,14].
Glycemic indices may also be classified as long-, intermediate-, or short-term glycemic indicators. The utility of A1c as a long-term glycemic indicator resides in the long half-life of erythrocytes (approximately 120 days). Consequently, A1c reflects glycemic status over the past 2 to 3 months. For intermediate-term glycemic indices, glycated serum proteins and albumin were introduced to assess glycemic status over intermediate periods (2 to 4 weeks) that reflect the half-lives of the respective molecules in the serum. GA, with a half-life of 12 to 19 days, would be an excellent glycation index of recent ambient glycemia [15]. Together, GA and FA levels may act as a powerful monthly management tool for diabetes. The indices 1,5-AG and glycated apo-B could serve as short-term glycemic indicators. Due to the short circulating half-life of LDLs (approximately 3 to 5 days), glycated LDL level reflects mean glycemia over approximately the preceding week [9]. The shortest index may be 1,5-AG. Because of the competitive inhibition of 1,5-AG reabsorption in the kidney tubule by glucose, 1,5-AG levels in the blood may respond with high sensitivity within 24 hours [11], reflecting even transient elevation of glucose within a few days [12]. Stettler et al. [16], however, suggested that the practical utility of 1,5-AG lies in its strong association with 2-hour postprandial glucose values in the preceding 2 weeks. From the perspective of quality of glucose control, another use of 1,5-AG in conjunction with A1c was in patients with moderate or good glucose control [12]. However, a study on the relationships between continuous glucose monitoring system data and the glycemic indices reported that GA levels more strongly correlate with plasma glucose levels and the glucose fluctuation index than those of A1c or 1,5-AG, especially in subjects with poor glucose control [17].
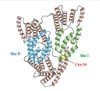
Albumin, synthesized mainly in the liver, is the most abundant of the plasma proteins, representing more than 80% of the total molecules and 50% to 60% of the total protein in the plasma of normal, healthy individuals [18]. Structurally, albumin is made up of 585 amino acids and contains 35 important cysteine residues that, except for Cys-34, form disulfide bridges that contribute to the overall tertiary structure of the protein (Fig. 1). Functionally, it exerts a wide variety of physiological and pharmacological functions, including regulation of colloid osmotic pressure of plasma due to its low molecular weight (67 kDa); transportation of hormones, fatty acids, drugs and metabolites due to its tertiary structure; and regulation of microvascular permeability, antioxidant activity, anti-thrombotic activity, and anti-inflammatory activity [10,19].
The antioxidant role of albumin has been underappreciated in diabetes. In general, a large proportion of the antioxidant properties of total serum can be attributed to albumin [20]. Bourdon and Blache [21] have shown that more than 70% of the free radical-trapping activity of serum, as assayed using the free radical-induced hemolysis test, was due to human serum albumin. In sites of inflammation, augmented albumin concentrations have been found to exert potent antioxidant activity [22]. Thus, human serum albumin is beneficial in patients with a variety of disorders by limiting oxidative damage. The antioxidant properties of albumin mainly arise from limiting reactive oxygen species (ROS) production by ligand-binding capacities as well as scavenging ROS through free radical-trapping activity. Regarding ligand-binding capacities, albumin easily binds molecules, such as metals ions, fatty acids, etc. Free Cu(II) and Fe(II) are metal ions that are very potent generators of ROS upon reacting with oxygen. They also interact with hydrogen peroxide, leading to formation of the deleterious hydroxyl radical via the Fenton reaction. When bound to albumin, copper and iron are less likely to be involved in the Fenton reaction [20]. The predominant contribution of albumin as an antioxidant in plasma might be due to several residues with antioxidant activities. As previously described, albumin consists of 585 amino acids and contains 6 methionine (Met) residues and 35 cysteine (Cys) residues. Of the 35 cysteine residue sites in albumin, Cys-34 is the only free cysteine residue not involved in disulfide bond formation, which endows albumin with its potent antioxidant activity. In reduced form, Cys-34 is able to not only scavenge hydroxyl radicals [23] but also covalently bind drugs [24]. Because of the abundance of albumin in plasma, a single reduced Cys-34 is enough to form the largest pool of thiols in the blood, contributing to powerful antioxidant effects. Six Met residues in human albumin represent an oxidation-sensitive amino acid. Levine et al. [25] suggested that the oxidation and reduction cycle of Met residues could serve as a ROS scavenging system to protect proteins from extensive modification.
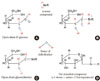
It is inevitable for proteins and lipids in the body to be exposed to hyperglycemia-related altering factors or reactions, whether enzymatic or non-enzymatic. Glycation and oxidation are the most common major non-enzymatic mechanisms. Glycation (sometimes called non-enzymatic glycosylation) is a simple process whereby excess sugar molecules, such as fructose or glucose, attach themselves to otherwise normal protein or lipid molecules in the blood without enzymatic intervention [26]. Monosaccharides inherently possess different glycation activities; it is known that galactose and fructose have approximately 10 times the glycation activity of glucose [27]. Concerns about glycation in diabetes arise from the fact that a reducing sugar has the potential to induce the glycation and impair the function of several proteins. Because all proteins are vulnerable to glycation, this impairment may have profound effects. Products of glycation can be classified into early or advanced products. Initially, a reversible, unstable Schiff base is formed from the attachment of glucose or derivatives with free amine groups of albumin (reversible glycation, 1 to 2 weeks of glycation; Fig. 2A and B), leading to formation of a stable fructosamine residue (ketoamine) through the removal of water (Fig. 2C). Rearrangement of this compound finally yields the irreversible Amadori compound (irreversible glycation, 6 to 8 weeks of glycation) (Fig. 2D) [28-30]. This is the early glycation process and is also known as the Maillard reaction. Advanced modifications in these early stage glycation products (Amadori adducts), such as rearrangement, oxidation, polymerization, and cleavage give rise to irreversible conjugates, called advanced glycation end products (AGE) [31].
Although GA testing may initially be viewed as an adjunct to A1c in the management of diabetes, its immediate applications are apparent as monthly tool for diabetes management. GA offers a similar reference discrepancy as A1c with a half-life on the order of 3 weeks rather than the 3 to 4 months of A1c. It also allows physicians to achieve better glycemic control and finally results in a superior index to use for reducing diabetic micro- and macrovascular complications. Amidst the ongoing debates about the interpretation of A1c, there is no consensus on a suitable cutoff value for A1c across different racial or ethnic populations. In a recent study on racial differences in glycemic markers, Selvin et al. [32] indicated that differences between African-American and Caucasian population with respect to GA, FA, and 1,5-AG levels parallel the differences in A1c among both non-diabetic and diabetic subjects. This result might imply a similarity between GA and A1c on racial and ethnic differences.
GA is now gaining popularity as an index of glycemic control during intensive treatment [33]. Won et al. [34] recently showed a significant direct correlation between changes in GA over a 3-week period and those in A1c over a 3-month period after intensified substitution of anti-diabetic drugs (oral hypoglycemic agent-based [r=0.735, P<0.01] and insulin-based [r=0.778, P<0.01]). A prospective, randomized, controlled trial has shown that initiation of once-daily basal insulin or biphasic insulin analogue followed by an intensified twice-daily biphasic insulin analogue for non-respondent subjects with greater than 20% GA at 3 weeks after the initiation of therapy yielded a significantly higher A1c reduction with guidance of interim GA values [35]. Thus, the use of GA as an intermediate-term index contributed in a guiding role to the control of glycemia. In addition, if the proper management of gestational diabetes or type 1 or 2 diabetes during pregnancy was found to be monthly diabetes management, GA could act as a standard glycation index. Thus, well-designed clinical studies for subjects with gestational diabetes are needed to determine the optimal glucose monitoring interval benefiting pregnant subjects and their offspring.
Even after proof of concept that intermediate-term glycemic indicators are beneficial and effective in achieving better glycemic control, leading to reduced diabetic complications through guiding glucose control, questions could be raised about which kind of glycemic marker would best satisfy the aforementioned unmet needs. As described previously, FA has physiologic disadvantages in that its levels are strongly influenced by the concentration of serum proteins and low-molecular-weight substances coexisting in the plasma [6,7]. FA has been scrutinized by the Food and Drug Administration in the United States, and a few clinical studies with mixed results were reported [36]. These clinical and practical data have led to its flagging popularity recently. During pregnancy, 1,5-AG is not a suitable parameter for glycemia management due to unstable renal hemodynamics [36].
Several studies have reported discrepancies in A1c as a standard indicator for glycemic control in patients with diabetes-associated pathologies. The diseases and pathological conditions in which GA is a more accurate glycation index might be diabetic subjects with hemolytic anemia, end-stage renal disease (ESRD), and iron deficiency. GA may also be more accurate for diabetic subjects during pregnancy [6,10,33,36]. Of the numerous pathologic conditions, studies targeting diabetic patients with ESRD have been well-designed and frequently conducted. Because of an altered erythrocyte lifespan caused by renal anemia, A1c measurements are low with respect to glycemic status in patients with ESRD. These findings issued concerns that physicians may underestimate and under-treat hyperglycemia in patients with chronic renal failure [36]. There is, however, no relevant evidence established by randomized controlled studies demonstrating that GA is better in preventing morbidity and mortality than A1c in this high-risk diabetic population. Though there is a lack of studies of abnormal albumin homeostasis in patients with ESRD, it is not difficult to imagine that there are potential fluctuations in GA in this population. In these regards, Mehrotra et al. [37] stated that it might be more reasonable to solve this drawback by obtaining consensus on different cutoffs for A1c in subjects with end-stage renal disease.
In contrast to A1c, GA itself is strongly involved in the development of major diabetic complications, including arterial stiffening, peripheral vascular calcification, nephropathy, retinopathy, and Alzheimer's disease [10]. Notwithstanding these merits, two important questions need to be answered through further prospective studies for GA to be considered a standard glycation index in these populations. First, evidence that changes in GA show better association with time-dependent proportional morbidities and mortality than those of A1c is required. Second, quarterly GA measurements need to assist physicians in achieving and maintaining better glycemic control and result in reducing diabetic complications in these populations.
Despite a very strong relationship between A1c and GA, some large discrepancies between the two values exist under certain physiologic and pathologic conditions. Because A1c and GA levels are dependent on the lifespan of erythrocytes and half-life of serum albumin, respectively, variability in the lifespan of erythrocytes and half-life of albumin between individuals could account for some unexplained GA/A1c ratio [38]. Physiologically, the rate of the nonenzymatic-glycation process on protein in vivo is approximately nine times faster than that of human hemoglobin [39], resulting in a ten-fold greater glycation reaction speed in albumin than in hemoglobin [40]. The conventional conversion of GA levels to A1c levels is often performed using the relationship A1c=GA/3, which is empirical and has a statistical basis [38]. Clinical reports in Korea and Japan have shown a wide range of mean ratios of GA to A1c (from 2.0 to 4.0) [34,38,41-44]. These differences in mean ratios of GA to A1c may originate from the heterogeneity of the study population, especially for A1c levels and stable or unstable status of glucose excursions. The mean GA/A1c ratio of those who participated in a periodic health examination who were mostly free of diabetes was 2.68 [42]. However, in studies targeting type 2 diabetics, including those with unstably controlled A1c with >0.5% fluctuation in A1c across 6 months, serum GA levels increased by 3% to 4% for every 1% increase in serum A1c levels [33,38]. Therefore, that GA/A1c ratio increased along with higher A1c may be attributable to more marked increases in GA levels than A1c levels in subjects with poorly controlled diabetes. Even among similar A1c levels, GA better reflected postprandial hyperglycemia [6,33,45], which is mainly caused by inadequate or dysfunctional endogenous insulin secretion. Koga et al. [41] measured GA and A1c in type 1 and 2 diabetes and found that the GA/A1c ratio was significantly higher in type 1 diabetic patients, in which glucose levels fluctuate over wider ranges, than in type 2 diabetic patients. Furthermore, Kim et al. [44] demonstrated that insulin secretory functions, such as HOMA-β and insulinogenic index, but not insulin resistance, associates with the ratio of GA to A1c in type 2 diabetics. The ratio of GA to A1c is increased as insulin secretory function is diminished. The reason behind these findings may lie in that fact that postprandial insulin secretory dysfunction in response to meal loading causes postprandial hyperglycemia, which directly leads to an augmented GA/A1c ratio. In a clinical view of this phenomenon, a physician could interpret the higher GA/A1c ratio as a greater postprandial glucose fluctuation due to deteriorating insulin secretory function. By assessing the meaning of the GA/A1c ratio, physicians may clinically relate the relevance of the higher ratio in situations of similar mean glucose levels. Because elevated levels of GA induce irreversible vascular damage in diabetes mellitus, further studies on the clinical relevance of higher GA/A1c ratios on diabetic complications would satisfy the unmet need of biologic impact of GA on cell pathophysiology. Thus, serial results guide its usefulness for management of these patients in clinical settings.
However, some physiological variables, such as body mass index or age, could affect GA levels. Also, in certain pathologic conditions affecting albumin metabolism, such as thyroid dysfunction, nephrotic syndrome, or liver cirrhosis, the ratio of GA to A1c should be interpreted with caution [10,46]. We summarized the clinical implication of GA/A1c (Table 1).
Despite the potential benefits of GA, on the lack of normal reference data on GA might limit its use as a diagnostic tool for diabetes. In contrast to A1c, GA levels are not influenced by sex [42,47]. In 2006, only the Japan Diabetes Society reported the reference intervals of GA (12.3% to 16.9%) in a normal population [42]. Recently, Hiramatsu et al. [48] reported reference intervals of GA in 676 healthy Japanese pregnant women of 11.5% to 15.7%. Furusyo et al. [42] measured fasting plasma glucose, A1c, and GA in 1,575 mostly non-diabetic Japanese subjects. Using receiver operating characteristic curve (ROC) analysis, GA ≥15.5% was optimal for predicting diabetes with a sensitivity of 83.3% and a specificity of 83.3%. Another Chinese study enrolled 1,971 outpatient subjects who underwent a 75 g oral glucose tolerance test and GA measurement and found a GA value of 17.1% to be an optimal cutoff in Chinese subjects using ROC analysis with 76.82% sensitivity and 76.89% specificity for the diagnosis of diabetes [49].
As previously described, GA is a more accurate glycation index to control glucose in certain diabetes-associated conditions, and further studies should be directed to assess the optimal cutoff value and clinical relevance in diabetic subjects with hemolytic anemia, ESRD, iron deficiency, and pregnancy.
In understanding the biologic impact of GA on diabetes and its complications, one should bear in mind that GA is a precursor of AGEs [50]. As previously described, high levels of glucose induce non-enzymatic glycation reactions between glucose and derivatives with free amine groups of albumin. These reactions successively produce a reversible unstable Schiff base, a stable fructosamine residue (ketoamine), an irreversible Amadori compound, and ultimately irreversible conjugates, called AGEs. Elevated levels of GA and AGEs are known to be actively involved in the development of diabetic vascular complications, such as retinopathy, nephropathy, neuropathy, and coronary artery disease [50-53]. Because of the approximately 17-day half-life of GA, GA may not reach the total levels of AGEs. However, GA is directly associated with the production of ROS, resulting in cell injury. Experimental endothelial cell damage due to ROS showed high clinical relevance in severity and extent of coronary artery disease and arterial stiffening in hemodialysis patients with type 2 diabetes [50,54,55].
To accept that GA is a pathogenic protein, one must also keep in mind that albumin contributes a large proportion of the antioxidant activity of total serum [20]. The antioxidant effects of albumin are due to limiting ROS production and scavenging ROS, as described previously. Glycation-induced modifications have a considerable impact on albumin functional properties, which can be related to alterations of its conformation [10,56]. Moreover, the sole residues prone to glycation in albumin are lysine, arginine, and cysteine, which is attributed to their highly nucleophilic characteristics [10]. Thus, glycation may block the powerful antioxidant properties of the Cys-34 residue. In addition, glycation is usually associated with oxidative modifications, which affect early stage glycation products. The simultaneous or combined reaction of oxidation during glycation is termed glycoxidation [10,57]. This oxidative state has been revealed through both an increase in carbonylated protein levels and a decrease in the reduced state of Cys-34 [10,58,59]. Therefore, the glycation of albumin brings about impaired antioxidant capacities partly via losing the free sulphydryl group of Cys-34 and partly via the pathologic effects of glycoxidation itself on endothelial and mesangial cells and macrophages. Furthermore, GA suppresses glucose-induced insulin secretion, impairing glucose metabolism in rat pancreatic β-cells [60]. These findings support and explain the strong involvement of GA in the development of major diabetic complications and the identification of people at risk for microvascular conditions.
Initially, the utility of GA was viewed as an adjunct to A1c for diabetes management but is now gaining popularity in month-based management of diabetes and diabetes-associated pathologies, such as hemolytic anemia, ESRD, and iron deficiency, as well as pregnancy. In addition, albumin is a pivotal antioxidant in human serum. Therefore, glycation and accompanied oxidation of albumin leads to a loss of antioxidant activity and generates an atherogenic protein in diabetes. This could explain why higher level GA related with vascular complication and diminished insulin secretory function. In conclusion, GA appears to have potential as a glycation index in diagnosing diabetes, guiding parameters after intensified medication, evaluating insulin secretory dysfunction along with glucose changes and predictor of diabetic complications.
Figures and Tables
ACKNOWLEGMENTS
We thank Hye Kyung Kim of Yonsei University Medical Library, for helping the authors in the preparation of this manuscript.
References
1. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999. 281:2005–2012.
2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008. 359:1577–1589.
3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012. 35:Suppl 1. S64–S71.
5. True MW. Circulating biomarkers of glycemia in diabetes management and implications for personalized medicine. J Diabetes Sci Technol. 2009. 3:743–747.
6. Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010. 57:751–762.
7. Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987. 33:2153–2163.
8. Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001. 56:1–21.
9. Lyons TJ, Baynes JW, Patrick JS, Colwell JA, Lopes-Virella MF. Glycosylation of low density lipoprotein in patients with type 1 (insulin-dependent) diabetes: correlations with other parameters of glycaemic control. Diabetologia. 1986. 29:685–689.
10. Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011. 93:645–658.
11. Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark ): a short-term glycemic marker. Diabetes Technol Ther. 2003. 5:355–363.
12. Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006. 29:1214–1219.
13. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability: does it matter? Endocr Rev. 2010. 31:171–182.
14. Kim MJ, Jung HS, Hwang-Bo Y, Cho SW, Jang HC, Kim SY, Park KS. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. Epub 2011 Jun 18. DOI: 10.1007/s00592-011-0302-0.
15. Cohen MP, Clements RS. Measuring glycated proteins: clinical and methodological aspects. Diabetes Technol Ther. 1999. 1:57–70.
16. Stettler C, Stahl M, Allemann S, Diem P, Schmidlin K, Zwahlen M, Riesen W, Keller U, Christ E. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008. 31:1534–1535.
17. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, Sada Y, Ishii S, Kondo A, Murakami K, Katabami T, Tanaka Y. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J. 2010. 57:135–140.
18. Evans TW. Review article: albumin as a drug: biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002. 16:Suppl 5. 6–11.
19. Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. 2011. 22:355–363.
20. Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008. 582:1783–1787.
21. Bourdon E, Blache D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. 2001. 3:293–311.
22. Halliwell B. Albumin: an important extracellular antioxidant? Biochem Pharmacol. 1988. 37:569–571.
23. Carballal S, Alvarez B, Turell L, Botti H, Freeman BA, Radi R. Sulfenic acid in human serum albumin. Amino Acids. 2007. 32:543–551.
24. Kragh-Hansen U, Chuang VT, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull. 2002. 25:695–704.
25. Levine RL, Berlett BS, Moskovitz J, Mosoni L, Stadtman ER. Methionine residues may protect proteins from critical oxidative damage. Mech Ageing Dev. 1999. 107:323–332.
26. Ahmed N, Furth AJ. Failure of common glycation assays to detect glycation by fructose. Clin Chem. 1992. 38:1301–1303.
27. McPherson JD, Shilton BH, Walton DJ. Role of fructose in glycation and cross-linking of proteins. Biochemistry. 1988. 27:1901–1907.
28. Hogan M, Cerami A, Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide: role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992. 90:1110–1115.
29. Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990. 173:932–939.
30. Rubenstein DA, Maria Z, Yin W. Glycated albumin modulates endothelial cell thrombogenic and inflammatory responses. J Diabetes Sci Technol. 2011. 5:703–713.
31. Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-kappa B and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta 1 production in macrophage RAW cells. J Lab Clin Med. 2003. 141:242–249.
32. Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011. 154:303–309.
33. Lee EY, Lee BW, Kim D, Lee YH, Kim KJ, Kang ES, Cha BS, Lee EJ, Lee HC. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol. 2011. 48:167–172.
34. Won HK, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. Reduction in glycated albumin can predict change in HbA1c: comparison of oral hypoglycaemic agent and insulin treatments. Diabet Med. 2012. 29:74–79.
35. Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011. 65:1076–1084.
36. Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008. 2:1114–1121.
37. Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol. 2011. 6:1520–1522.
38. Tahara Y. Analysis of the method for conversion between levels of HbA1c and glycated albumin by linear regression analysis using a measurement error model. Diabetes Res Clin Pract. 2009. 84:224–229.
39. Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983. 258:6142–6146.
40. Iberg N, Fluckiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986. 261:13542–13545.
41. Koga M, Murai J, Saito H, Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010. 33:270–272.
42. Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011. 54:3028–3036.
43. Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007. 54:139–144.
44. Kim D, Kim KJ, Huh JH, Lee BW, Kang ES, Cha BS, Lee HC. The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin Endocrinol (Oxf). Epub 2011 Dec 9. DOI: 10.1111/j.1365-2265.2011.04312.x.
45. Koga M, Murai J, Saito H, Mukai M, Matsumoto S, Kasayama S. Glycated albumin levels are higher relative to glycated haemoglobin levels in gastrectomized subjects. Ann Clin Biochem. 2010. 47(Pt 1):39–43.
46. Kim MK, Kwon HS, Baek KH, Lee JH, Park WC, Sohn HS, Lee KW, Song KH. Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care. 2010. 33:2546–2548.
47. Schaefer EJ, Audelin MC, McNamara JR, Shah PK, Tayler T, Daly JA, Augustin JL, Seman LJ, Rubenstein JJ. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001. 88:1129–1133.
48. Hiramatsu Y, Shimizu I, Omori Y, Nakabayashi M. Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J. 2012. 59:145–151.
49. Ma XJ, Pan JM, Bao YQ, Zhou J, Tang JL, Li Q, Xiang KS, Jia WP. Combined assessment of glycated albumin and fasting plasma glucose improves the detection of diabetes in Chinese subjects. Clin Exp Pharmacol Physiol. 2010. 37:974–979.
50. Lu L, Pu LJ, Zhang Q, Wang LJ, Kang S, Zhang RY, Chen QJ, Wang JG, De Caterina R, Shen WF. Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2009. 206:540–545.
51. Rodino-Janeiro BK, Gonzalez-Peteiro M, Ucieda-Somoza R, Gonzalez-Juanatey JR, Alvarez E. Glycated albumin, a precursor of advanced glycation end-products, up-regulates NADPH oxidase and enhances oxidative stress in human endothelial cells: molecular correlate of diabetic vasculopathy. Diabetes Metab Res Rev. 2010. 26:550–558.
52. Lee BW, Ihm J, Kang JG, Choi MG, Yoo HJ, Ihm SH. Amadori-glycated albumin-induced vascular smooth muscle cell proliferation and expression of inhibitor of apoptosis protein-1 and nerve growth factor-gamma. Biofactors. 2007. 31:145–153.
53. Li Y, Wang S. Glycated albumin activates NADPH oxidase in rat mesangial cells through up-regulation of p47phox. Biochem Biophys Res Commun. 2010. 397:5–11.
54. Kumeda Y, Inaba M, Shoji S, Ishimura E, Inariba H, Yabe S, Okamura M, Nishizawa Y. Significant correlation of glycated albumin, but not glycated haemoglobin, with arterial stiffening in haemodialysis patients with type 2 diabetes. Clin Endocrinol (Oxf). 2008. 69:556–561.
55. Kim HM, Kim KJ, Moon JH, Lee HJ, Chae MK, Chang HJ, Kang ES, Cha BS, Lee HC, Kim YJ, Lee BW. Association between EPCs count and rate of coronary revascularization in asymptomatic type 2 diabetic patients. Acta Diabetol. Epub 2011 Dec 13. DOI: 10.1007/s00592-011-0360-3.
56. Bourdon E, Loreau N, Lagrost L, Blache D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic Res. 2005. 39:15–20.
57. Lyons TJ. Glycation and oxidation: a role in the pathogenesis of atherosclerosis. Am J Cardiol. 1993. 71:26B–31B.
58. Faure P, Troncy L, Lecomte M, Wiernsperger N, Lagarde M, Ruggiero D, Halimi S. Albumin antioxidant capacity is modified by methylglyoxal. Diabetes Metab. 2005. 31:169–177.
59. Chesne S, Rondeau P, Armenta S, Bourdon E. Effects of oxidative modifications induced by the glycation of bovine serum albumin on its structure and on cultured adipose cells. Biochimie. 2006. 88:1467–1477.
60. Shiraki T, Miura Y, Sawada T, Okada T, Sakuraoka Y, Muto T, Kubota K. Glycated albumin suppresses glucose-induced insulin secretion by impairing glucose metabolism in rat pancreatic beta-cells. Nutr Metab (Lond). 2011. 8:20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download