Abstract
Objective
Systemic sclerosis is a connective tissue disease characterized by vasculopathy, excessive accumulation of extracellular matrix, and fibrosis of the skin and internal organs. The dietary flavonoid apigenin has been shown to reduce expression of the myofibroblast phenotype and to inhibit contraction of collagen gels. We investigated the effect of apigenin on the prevention and treatment of a modified bleomycin-induced animal model of scleroderma.
Methods
Recently, we successfully induced scleroderma by weekly subcutaneous injections of bleomycin using a thermo-reversible combination gel composed of low molecular weight methylcellulose. A weekly subcutaneous injection of methylcellulose gel loaded with bleomycin induced focal skin fibrosis on the back skin and fibrotic phenotype of lung tissue in mice. The histologic examination of skin and lungs, collagen assay of lungs, and expression of connective tissue growth factor were investigated.
Systemic sclerosis (SSc) is a connective tissue disease characterized by vasculopathy, excessive accumulation of extracellular matrix (ECM), and fibrosis of the skin and internal organs (1). Many cytokines such as transforming growth factor beta (TGF-β), platelet-derived growth factor, tumor necrosis factor, interleukin (IL)-4, IL-6, and IL-13 have been well-known to be involved in the pathogenesis of SSc. TGF-β is the most powerful profibrogenic stimulus to fibroblasts, where these effects of TGF-β are mainly mediated by connective tissue growth factor (CTGF) (2). Until now no single agent has been convincingly shown to be an effective treatment, and the pathogenesis of SSc remains unknown.
The largest class of polyphenols, flavonoids, has a variety of biological properties (3). The common non-toxic and non-mutagenic dietary flavonoid, apigenin (4), is abundant in fruits such as grapes and cherries, and in vegetables such as parsley, onions, and broccoli (5). It affects many cellular processes such as cell proliferation, migration, tumor growth, and fibrosis (3,5). Ricupero et al. (5) reported that apigenin inhibited the proliferation of myofibroblasts and expression of α1 (1) collagen and α-smooth muscle actin (α-SMA). Moreover, apigenin decreased TGF-β-induced up-regulation of SMA mRNA probably by inhibiting Akt phosphorylation. Recently we (6) demonstrated that physiological concentrations of apigenin inhibited endothelin-1-induced contraction of collagen gels in an in vitro model of ECM remodeling (7).
There are several animal models of SSc. In particular, a bleomycin (BLM)-induced SSc model involving daily subcutaneous injection of BLM into the back skins of mice of susceptible strains is widely used. BLM-treated mice showed not only all histological characteristics of human scleroderma skin, but also lung fibrosis. Perivascular cellular infiltration consisted of CD4+ T cells, mast cells, and macrophages (8). However, daily injection is time-consuming and inconvenient. Recently, we successfully induced SSc by weekly subcutaneous injections of BLM using a thermoreversible combination gel composed of low molecular weight methylcellulose (LMw MC) (9,10). The objective of the present work was to investigate the therapeutic efficacy of physiological concentrations of apigenin in this modification of the BLM-induced SSc model.
The preparation of LMw MC gels was described in detail previously (10). BLM was loaded into the gels by mixing with 4% LMw MC/4.5% ammonium sulfate (Sigma-Aldrich, St. Louis, MO, USA) in PBS. In vitro release of BLM from MC gels was assessed according to the previous protocol. After removing the hair, 50 µL of MC-BLM mixture or MC-PBS was injected subcutaneously into the back skin of 7-week-old C3H/He mice at weekly intervals.
Approval of the Institutional Animal Care and Use Committee was obtained for the animal experiments. Apigenin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in DMSO (Calbiochem, San Diego, CA, USA). Based on the previous reports (11,12), we decided to use a concentration of 10 mg/kg of apigenin for intraperitoneal (IP) injection. The mice were divided into 3 groups of 10 mice per group: [1] PBS injection into the skin and no IP injection, [2] MC-BLM injection into the skin and IP injection of 8% DMSO, and [3] MC-BLM injection into the skin and IP injection of 10 mg/kg of apigenin with 8% DMSO. Apigenin was injected everyday intraperitoneally from a week before the 1st injection of MC-BLM gel. General appearance and body weight were monitored weekly immediately before injecting the MC-BLM gel. Unfortunately, however, after 2 weeks and 3 weeks of daily IP injection of apigenin, three mice and one mouse, respectively, died. However, none of the mice receiving a daily IP injection of 8% DMSO died. We evaluated dermal thickness, inflammation and grade of fibrosis of skin tissue in group 1 (n=4), group 2 (n=4), and group 3 (n=3), because the experiment was terminated prematurely (4 weeks after the 1st injection of apigenin) and we selected just a few of the mice for this preliminary study. Thereafter, we decided to decrease the concentration of IP apigenin to 2.5 mg/kg and 1.0 mg/kg. Mice were assigned to four groups of 9 mice per group [Control; PBS injection into skin and no IP injection, MC-BLM group; MC-BLM gel injection into skin and IP injection of 8% DMSO, Apgenin 1.0 or 2.5 group; MC-BLM gel injection into skin and IP injection of apigenin (1.0 mg/kg or 2.5 mg/kg) with 8% DMSO]. The subsequent general appearance and body weight of the mice were stable in all the groups (data not shown), and only one mouse injected IP with 2.5 mg/kg of apigenin died after 3 weeks of apigenin injection.
Four weeks after the first injection, the mice were killed and skin tissue around the injection site was obtained for histological examination. Skin specimens were fixed with 10% formalin solution and embedded in paraffin. Two separate skin specimens were sectioned at 4 µm thickness, and 5 consecutive sections at each site were stained with hematoxylin and eosin (H&E), Masson-Trichrome (M-T), and monoclonal antibody against α-SMA. Dermal thickness, the intensity of fibrosis, the extent of mononuclear cell infiltration, and numbers of α-SMA-positive fibroblastic cells, were evaluated as described in detail previously (10). The mean values at two sites were averaged for statistical analysis.
The left lung was ligated at the level of the main bronchus, excised at the hilum, and immediately frozen in liquid nitrogen for collagen assay. The right lung was fixed with 10% formaldehyde in a neutral-buffered solution for 48 hours and embedded in paraffin. Sagittal sections, 2 µm thick, were cut and stained with H&E and M-T stain. The total area of the lung sections was used for microscopic scoring of fibrosis. Lung fibrosis was graded according to the method described previously by Ashcroft et al. (13), and described in detail elsewhere (14). The grade of lung fibrosis was scored by evaluating 10 randomly chosen regions per section at ×200 magnification by an experienced pulmonologist (Kim SH) who was blinded to the treatment groups. The mean of the scores from all examinations was taken as the fibrotic score.
Lung collagen content was determined by assaying total soluble collagen using the Sircol™ soluble collagen assay (Biocolor, Carrickfergus, Co., Antrim, UK) as described previously (15).
Sera were obtained by cardiac puncture when the mice were killed, and stored at -80℃. Serum CTGF levels were assessed using a Quantikine CTGF Immunoassay Kit (R&D Systems, Inc., Minneapolis, MN, USA).
All data are expressed as means±standard deviations. The t-test or Mann-Whitney rank sum test was used to test the significance of differences between the negative control and the MC-BLM gel group. One way analysis of variance was used to test the significance of differences between the MC-BLM gel control and the two apigenin injection groups. p-values of less than 0.05 were considered statistically significant.
Preliminary study with IP injection of 10.0 mg/kg of apigenin (Figure 1).
Dermal thickness was significantly increased in the MC-BLM gel group (274.4±59.7 µm) compared to the PBS control group (188.0±34.5 µm), and fell slightly in the apigenin (10 mg/kg) IP group (229.7±13.0 µm), but the difference was not statistically significant. Skin fibrosis and inflammation grading were significantly increased in the MC-BLM gel group and decreased in the apigenin group (data not shown). The MC-BLM gel treatment was successful in inducing murine SSc but the concentration of apigenin injected seemed to be lethal in this setting.
Four weeks after the first MC gel injection (5 weeks after the 1st apigenin injection), we examined the histological changes around the injection sites on the skin of the mice. Dermal thickness, and semi-quantitative analysis of fibrosis and mast cell infiltration were significantly increased in the MC-BLM gel injection group compared to the MC-PBS injection group. The number of α-SMA-positive cells was larger in the MC-BLM group than the MC-PBS group but without statistical significance (6.0±1.8 vs. 5.0±0.7, p=0.141). A semi-quantitative analysis of inflammation revealed no significant difference between the MC-PBS and MC-BLM group. These results show that this murine model of scleroderma is effective, as reported previously. However, daily IP injection of 1.0 mg/kg or 2.5 mg/kg apigenin was not effective in preventing and treating the skin fibrosis in terms of dermal thickness, fibrosis, mast cell infiltration, or number of α-SMA-positive cells.
Histological evaluation of lung fibrosis revealed that BLM injection increased the fibrotic score (4.3±0.8) compared to the control group (3.7±1.1), but the difference was not statistically significant. Daily IP injection of apigenin (1.0 mg/kg or 2.5 mg/kg) resulted in a decrease of fibrotic score, but again the difference was not statistically significant (4.2±0.9 and 3.8±0.9 respectively).
Daily intra-peritoneal injection of 1.0 mg/kg or 2.5 mg/kg of apigenin starting a week before the bleomycin injections failed to prevent the development of skin fibrosis and reduce the fibrotic phenotype of skin and lung tissue.
The aim of this study was to investigate the effect of the dietary flavonoid apigenin on the prevention and treatment of the modified BLM-induced SSc murine model using MC gels developed by the authors. Apigenin is generally administered orally with the diet (0.2% or 0.05%) or intraperitoneally, and we chose IP injection to assure correct administration and to maximize therapeutic efficacy. Unfortunately, however, IP injection of 10 mg/kg of apigenin dissolved in DMSO killed three mice and one mouse, respectively, after two weeks and three weeks of the 1st injection of apigenin. On the other hand no mice died in the control DMSO IP injection group. Therefore we thought that a 10 mg/kg IP concentration of apigenin was toxic in the strain of mice used, although it has been reported that daily IP injection of 20 mg/kg of apigenin for as long as 52 weeks was non-toxic in a different mouse strain (11). In view of the results of this preliminary experiment, we decreased the concentration of apigenin to 1.0 mg/kg and 2.5 mg/kg and this may have been responsible for the negative results of our study. In the population of Holland the average intake of daily dietary apigenin is reported to be 1 mg (16). Moreover, after consumption of 64.2 mg of quercetin, the most abundant of the flavonoids ingested by the Dutch population, peak plasma concentration averaged 0.6 µmol/L (17). Furthermore, in a study of 20 Chinese students, the mean daily dietary intake and fasting plasma concentrations of apigenin were 4.23 mg/d and 10.62 nmol/L, respectively (18). Therefore, given the difference between humans and mice, we believe that the concentrations of apigenin (1.0 mg/kg and 2.5 mg/kg) used in this study were not too low, and that the plasma concentration of apigenin would have exceeded the level of apigenin found in humans and mice on normal diets, although direct comparison between oral intake and IP injection, and between different species, was not feasible.
We failed to demonstrate that IP injection of apigenin (1.0 mg/kg or 2.5 mg/kg) was effective in preventing and treating skin and lung fibrosis in the BLM-induced mouse model of SSc, although some in vitro experiments have supported a potential role of apigenin in the treatment of fibrosis. Further studies in other animal models of SSc, and long-term administration of apigenin with follow-up are called for.
Figures and Tables
Figure 1
Histological examination of skin: (A) Dermal thickness (rectangular blank bar, µm) on hematoxylin and eosin stain (×200), (B) Semi-quantitative analysis of fibrosis on Masson-Trichrome stain (×200), and (C) Semi-quantitative analysis of mast cell infiltration on toluidine blue stain (arrow indicated) (×400). In the MC-BLM group, dermal thickness, fibrosis, and mast cell infiltration were increased, compared to the MC-PBS control group, but intraperitoneal injection of apigenin (1.0 mg/kg and 2.5 mg/kg) did not decrease these changes in intraperitoneal apigenin groups. MC: methylcellulose gel, PBS: phosphate buffered saline, BLM: bleomycin, AP: apigenin.

Figure 2
Histological examination of the lung. Histological grading of lung tissue on Masson-Trichrome stain (upper panel: ×40, lower panel: ×200). In the MC-BLM group, the lung histological grade was increased, compared to the MC-PBS control group, but intraperitoneal injection of apigenin (1.0 mg/kg and 2.5 mg/kg) did not decrease these changes in intraperitoneal apigenin groups. MC: methylcellulose gel, PBS: phosphate buffered saline, BLM: bleomycin, AP: apigenin.
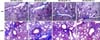
Table 1
Histological examination of skin and lung tissue, and collagen contents of lung

Control group; PBS into skin+No IP injection, MC-BLM group; BLM into skin+PBS IP injection, Apigenin 1.0 group; BLM into skin+AP (1.0 mg/kg) IP, Apigenin 2.5 group; BLM into skin+AP (2.5 mg/kg) IP, PBS: phosphate buffered saline, BLM: bleomycin, AP: apigenin, IP: intraperitoneal, α-SMA: α-smooth muscle actin. *p<0.05 vs. control, student T-test or Mann-Whitney rank sum test, †p<0.05 vs. MC-BLM group, one way ANOVA
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090023).
References
1. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988. 15:202–205.
2. Ihn H. Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci. 2008. 49:103–113.
3. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002. 22:19–34.
4. Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 2000. 3:447–451.
5. Ricupero DA, Poliks CF, Rishikof DC, Kuang PP, Goldstein RH. Apigenin decreases expression of the myofibroblast phenotype. FEBS Lett. 2001. 506:15–21.
6. Jun JB, Na YI, Kim TH, Yoo DH. Dietary flavonoid apigenin inhibits endothelin-1-induced contraction of collagen gel. Rheumatol Int. 2010. 30:1695–1697.
7. Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, et al. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol. 2001. 116:417–425.
8. Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999. 112:456–462.
9. Jin KM, Kim YH. Injectable, thermo-reversible and complex coacervate combination gels for protein drug delivery. J Control Release. 2008. 127:249–256.
10. Jun JB, Kim JK, Na YI, Jang SM, Paik SS, Kim YH. A convenient method for producing the bleomycin-induced mouse model of scleroderma by weekly injections using a methylcellulose gel. Rheumatol Int. 2012. 32:1443–1447.
11. Kang HK, Ecklund D, Liu M, Datta SK. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther. 2009. 11:R59.
12. Myoung HJ, Kim G, Nam KW. Apigenin isolated from the seeds of Perilla frutescens britton var crispa (Benth) inhibits food intake in C57BL/6J mice. Arch Pharm Res. 2010. 33:1741–1746.
13. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988. 41:467–470.
14. Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, et al. Blockade of the Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med. 2011. 223:45–54.
15. Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am J Respir Cell Mol Biol. 2003. 29:375–380.
16. Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993. 20:21–29.
17. Hollman PC, vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med. 1996. 21:703–707.
18. Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010. 103:249–255.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download