Abstract
Cardiac involvement is an important cause of mortality in patients with Churg-Strauss syndrome. The typical cardiac presentation of Churg-Strauss syndrome includes pericarditis, myocarditis, and cardiomyopathy. Endomyocardial fibrosis has rarely been described in patients with Churg-Strauss syndrome. We experienced a patient with Churg-Strauss syndrome who exhibited exertional dyspnea and endomyocardial fibrosis visualized as delayed enhancement on cardiac magnetic resonance imaging (MRI). After glucocorticoid treatment, the patient's symptom resolved, and the eosinophil count decreased to normal. Nine months later, the delayed-enhanced lesion on the cardiac MRI nearly disappeared. Here, we report a case of endomyocardial fibrosis in a patient with Churg-Strauss syndrome with a literature review.
REFERENCES
1). Churg J., Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951. 27:277–311.
2). Pagnoux C., Guilpain P., Guillevin L. Churg-Strauss syndrome. Curr Opin Rheumatol. 2007. 19:25–32.

3). Lanham J., Cooke S., Davies J., Hughes G. Endomyocardial complications of the Churg-Strauss syndrome. Postgrad Med J. 1985. 61:341–4.

4). Oh M., Lee J., Kwon N., Choi D. Churg-Strauss syndrome: the clinical features and long-term follow-up of 17 patients. J Korean Med Sci. 2006. 21:265–71.

5). Masi AT., Hunder GG., Lie JT., Michel BA., Bloch DA., Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990. 33:1094–100.

6). Sinico RA., Di Toma L., Maggiore U., Bottero P., Radice A., Tosoni C, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005. 52:2926–35.

7). Sable-Fourtassou R., Cohen P., Mahr A., Pagnoux C., Mouthon L., Jayne D, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005. 143:632–8.

8). Wassmuth R., Gobel U., Natusch A., Schneider W., Kettritz R., Dietz R, et al. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg-Strauss syndrome. J Card Fail. 2008. 14:856–60.

9). Alter P., Maisch B. Endomyocardial fibrosis in Churg-Strauss syndrome assessed by cardiac magnetic resonance imaging. Int J Cardiol. 2006. 108:112–3.

10). Moon J., Reed E., Sheppard M., Elkington A., Ho S., Burke M, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004. 43:2260–4.

11). Laissy JP., Messin B., Varenne O., Iung B., Karila-Cohen D., Schouman-Claeys E, et al. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest. 2002. 122:1638–48.
12). Pfeil A., Brehm B., Lopatta E., Neumann T., Schmidt P., Wolf G, et al. Acute chest pain, heart failure, and eosinophilia in a woman without coronary disease. Cardiovasc Intervent Radiol. 2009. 32:1272–4.

13). Baccouche H., Yilmaz A., Alscher D., Klingel K., Val-Bernal J., Mahrholdt H. Magnetic resonance assessment and therapy monitoring of cardiac involvement in Churg-Strauss syndrome. Circulation. 2008. 117:1745–9.

14). Ribi C., Cohen P., Pagnoux C., Mahr A., Arene J., Lauque D, et al. Treatment of Churg-Strauss syndrome without poor-prognosis factors: a multicenter, prospective, randomized, open-label study of seventy-two patients. Arthritis Rheum. 2008. 58:586–94.

15). Guillenvin L., Lhote F., Gayraud M., Cohen P., Jarrouse B., Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome: a prospective study in 342 patients. Medicine (Baltimore). 1996. 75:17–28.
Fig. 1.
Transthoracic echocardiography shows echogenic mass in the right ventricular apex (arrow) with right ventricularcavity obliteration and a moderate amount of pericardial effusion (arrowhead). The left ventricular cavity size and systolic function were normal (ejection fraction: 64%). (A) Parasternal long axis view (B) Four chambered view.
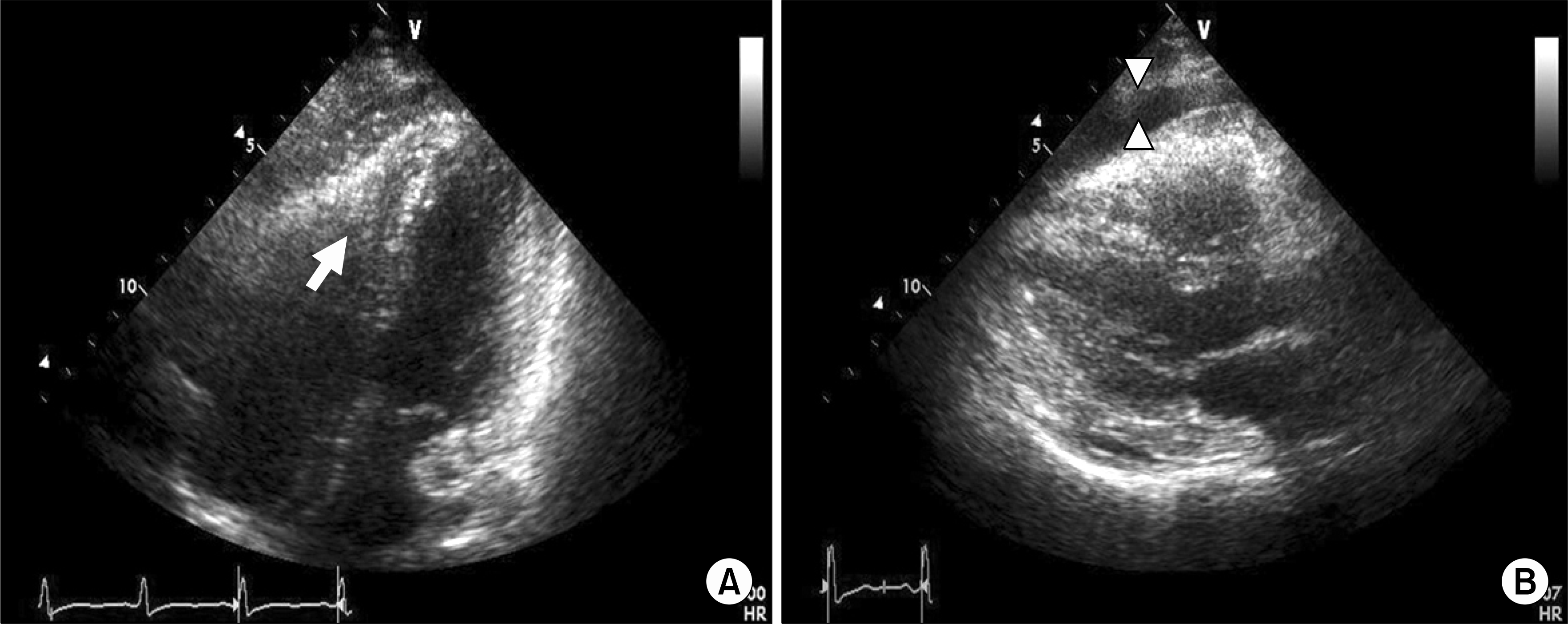
Fig. 2.
Magnetic resonance imaging (MRI). (A) Diffuse wall thickening and endomyocardial linear high signal intensity of the right ventricle (white arrows) with constricted ventricular cavity on T2-weighted MRI. (B) Delayed enhancement MRI shows endomyocardial enhancement of the right ventricle (arrowhead) and tiny low signal intensities (thrombus) (arrow) in the right ventricular cavity. (C) A 1-month follow-up image shows no significant interval change in the right ventricular apical obliteration and small right ventricular cavity on T2-weighted MRI. (D) A 9-month follow-up image shows a nearly disappeared delayed enhancement of the right ventricle (arrowhead) and absent thrombi in the right ventricular cavity (arrow).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download