1. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37:2129–2200. PMID:
27206819.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128:e240–e327. PMID:
23741058.
3. Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015; 17:544–558. PMID:
25999021.
4. Mebazaa A, Tolppanen H, Mueller C, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016; 42:147–163. PMID:
26370690.
5. Harjola VP, Mebazaa A, Čelutkienė J, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016; 18:226–241. PMID:
26995592.
6. Akiyama E, Van Aelst LN, Arrigo M, et al. East Asia may have a better 1-year survival following an acute heart failure episode compared with Europe: results from an international observational cohort. [Epub ahead of print]. Eur J Heart Fail. 2018.
7. Follath F, Yilmaz MB, Delgado JF, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med. 2011; 37:619–626. PMID:
21210078.
8. Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002; 287:628–640. PMID:
11829703.
9. Stevenson LW. Design of therapy for advanced heart failure. Eur J Heart Fail. 2005; 7:323–331. PMID:
15718172.
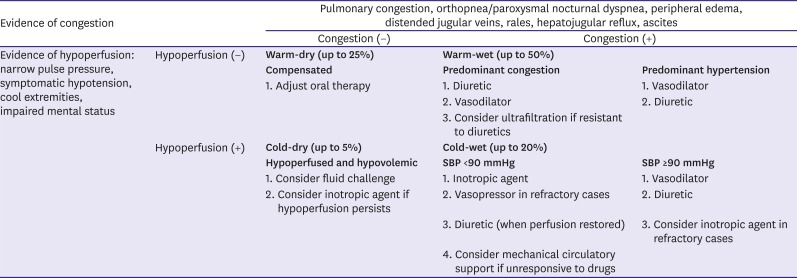
10. Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003; 41:1797–1804. PMID:
12767667.
11. Zannad F, Mebazaa A, Juillière Y, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006; 8:697–705. PMID:
16516552.
12. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008; 52:534–540. PMID:
18687247.
13. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009; 15:256–264. PMID:
19925503.
14. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992; 21:669–674. PMID:
1590605.
15. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39:119–177. PMID:
28886621.
16. Shaefi S, O'Gara B, Kociol RD, et al. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015; 4:e001462. PMID:
25559014.
17. Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009; 16:201–210. PMID:
19183402.
18. Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016; 37:2097–2104. PMID:
27174289.
19. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014; 21:843–852. PMID:
25176151.
20. Collins SP, Lindsell CJ, Storrow AB, Abraham WT. ADHERE Scientific Advisory Committee, Investigators and Study Group. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006; 47:13–18. PMID:
16387212.
21. Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991; 90:353–359. PMID:
1825901.
22. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35:3033–3073. PMID:
25173341.
23. Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997; 350:1349–1353. PMID:
9365448.
24. Lassus J, Gayat E, Mueller C, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013; 168:2186–2194. PMID:
23538053.
25. Felker GM, Mentz RJ, Teerlink JR, et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015; 17:1262–1270. PMID:
26333655.
26. Peacock WF 4th, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008; 358:2117–2126. PMID:
18480204.
27. Nikolaou M, Parissis J, Yilmaz MB, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013; 34:742–749. PMID:
23091203.
28. Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000; 35:681–689. PMID:
10716471.
29. Masip J, De Mendoza D, Planas K, Paez J, Sanchez B, Cancio B. Peripheral venous blood gases and pulse-oximetry in acute cardiogenic pulmonary oedema. Eur Heart J Acute Cardiovasc Care. 2012; 1:275–280. PMID:
24062917.
30. Branson RD, Johannigman JA. Pre-hospital oxygen therapy. Respir Care. 2013; 58:86–97. PMID:
23271821.
31. Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA. 2005; 294:3124–3130. PMID:
16380593.
32. Plaisance P, Pirracchio R, Berton C, Vicaut E, Payen D. A randomized study of out-of-hospital continuous positive airway pressure for acute cardiogenic pulmonary oedema: physiological and clinical effects. Eur Heart J. 2007; 28:2895–2901. PMID:
17967821.
33. Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ. Diuretics for heart failure. Cochrane Database Syst Rev. 2012; CD003838. PMID:
22336795.
34. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017; 50:1602426. PMID:
28860265.
35. Liesching T, Kwok H, Hill NS. Acute applications of noninvasive positive pressure ventilation. Chest. 2003; 124:699–713. PMID:
12907562.
36. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364:797–805. PMID:
21366472.
37. Mentz RJ, Kjeldsen K, Rossi GP, et al. Decongestion in acute heart failure. Eur J Heart Fail. 2014; 16:471–482. PMID:
24599738.
38. Cox ZL, Lenihan DJ. Loop diuretic resistance in heart failure: resistance etiology-based strategies to restoring diuretic efficacy. J Card Fail. 2014; 20:611–622. PMID:
24879974.
39. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol. 2012; 60:1906–1912. PMID:
23062527.
40. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012; 33:1787–1847. PMID:
22611136.
41. Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998; 351:389–393. PMID:
9482291.
42. Packer M, O'Connor C, McMurray JJ, et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017; 376:1956–1964. PMID:
28402745.
43. Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013; 381:29–39. PMID:
23141816.
44. Teerlink JR, Voors AA, Ponikowski P, et al. Serelaxin in addition to standard therapy in acute heart failure: rationale and design of the RELAX-AHF-2 study. Eur J Heart Fail. 2017; 19:800–809. PMID:
28452195.
46. Peacock WF, Chandra A, Char D, et al. Clevidipine in acute heart failure: Results of the A Study of Blood Pressure Control in Acute Heart Failure-A Pilot Study (PRONTO). Am Heart J. 2014; 167:529–536. PMID:
24655702.
47. Mebazaa A, Longrois D, Metra M, et al. Agents with vasodilator properties in acute heart failure: how to design successful trials. Eur J Heart Fail. 2015; 17:652–664. PMID:
26040488.
48. Peacock WF, Hollander JE, Diercks DB, Lopatin M, Fonarow G, Emerman CL. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008; 25:205–209. PMID:
18356349.
49. Mebazaa A, Motiejunaite J, Gayat E, et al. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail. 2018; 20:332–341. PMID:
28990358.
50. Mortara A, Oliva F, Metra M, et al. Treatment with inotropes and related prognosis in acute heart failure: contemporary data from the Italian Network on Heart Failure (IN-HF) Outcome registry. J Heart Lung Transplant. 2014; 33:1056–1065. PMID:
25049067.
51. Mebazaa A, Parissis J, Porcher R, et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011; 37:290–301. PMID:
21086112.
52. Flaherty JD, Bax JJ, De Luca L, et al. Acute heart failure syndromes in patients with coronary artery disease early assessment and treatment. J Am Coll Cardiol. 2009; 53:254–263. PMID:
19147042.
53. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010; 56:1071–1078. PMID:
20863950.
54. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37:267–315. PMID:
26320110.
55. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015; 36:2793–2867. PMID:
26320108.
56. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962. PMID:
27567408.
57. European Society of Cardiology (ESC). European Heart Rhythm Association (EHRA). 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. 2013; 15:1070–1118. PMID:
23801827.
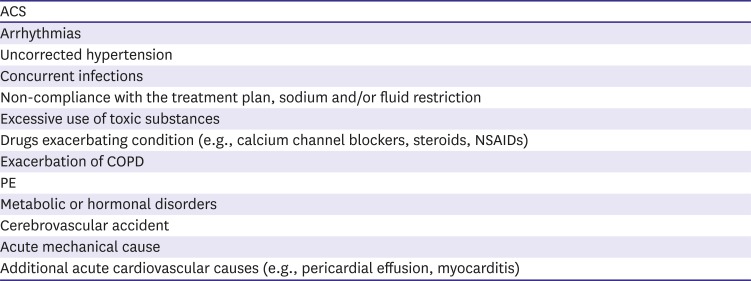
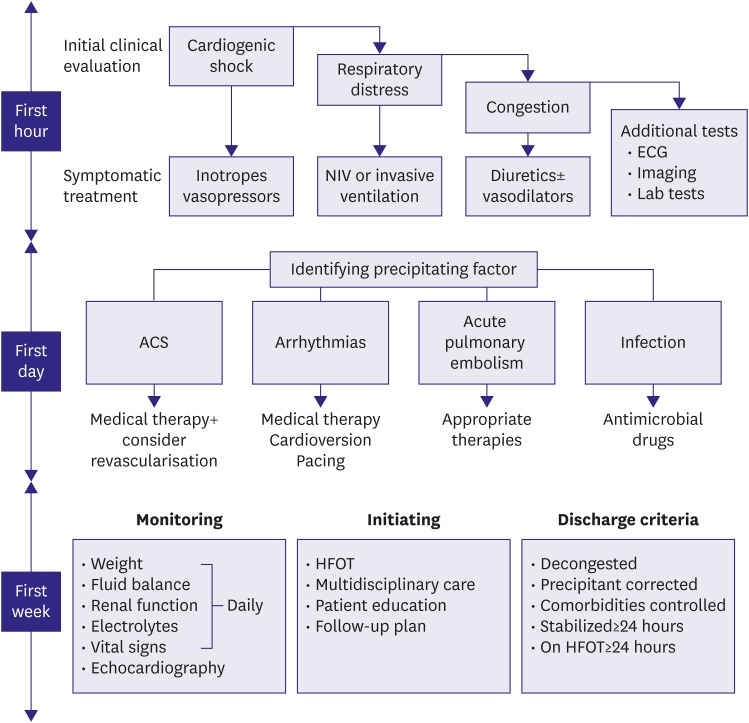
58. Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008; 168:847–854. PMID:
18443260.
59. Arrigo M, Tolppanen H, Sadoune M, et al. Effect of precipitating factors of acute heart failure on readmission and long-term mortality. ESC Heart Fail. 2016; 3:115–121. PMID:
27812386.
60. Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008; 36:1701–1706. PMID:
18496368.
61. Maisel A, Neath SX, Landsberg J, et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012; 14:278–286. PMID:
22302662.
62. Möckel M, Searle J, Maisel A. The role of procalcitonin in acute heart failure patients. ESC Heart Fail. 2017; 4:203–208. PMID:
28772049.
63. Demissei BG, Cleland JG, O'Connor CM, et al. Procalcitonin-based indication of bacterial infection identifies high risk acute heart failure patients. Int J Cardiol. 2016; 204:164–171. PMID:
26666342.
64. Fonarow GC, Adams KF, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005; 293:572–580. PMID:
15687312.
65. Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007; 13:599–608. PMID:
17923350.
66. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013; 309:355–363. PMID:
23340637.
67. Gheorghiade M, Peterson ED. Improving postdischarge outcomes in patients hospitalized for acute heart failure syndromes. JAMA. 2011; 305:2456–2457. PMID:
21673297.
68. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2015; 3:647–653. PMID:
26251094.
69. Khan SS, Gheorghiade M, Dunn JD, Pezalla E, Fonarow GC. Managed care interventions for improving outcomes in acute heart failure syndromes. Am J Manag Care. 2008; 14:S273–S286. PMID:
19166273.
70. Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005; 111:179–185. PMID:
15642765.
71. Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015; 17:501–509. PMID:
25820680.
72. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017; 377:2419–2432. PMID:
29083953.
73. Levy B, Bastien O, Bendjelid K, et al. Experts' recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015; 5:52.
74. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015; 36:1223–1230. PMID:
25732762.
75. Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011; 39:450–455. PMID:
21037469.
76. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010; 362:779–789. PMID:
20200382.
77. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367:1287–1296. PMID:
22920912.
78. Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013; 382:1638–1645. PMID:
24011548.
79. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014; 64:1407–1415. PMID:
25277608.
80. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67–119. PMID:
26320113.
81. Arrigo M, Mebazaa A. Understanding the differences among inotropes. Intensive Care Med. 2015; 41:912–915. PMID:
25605474.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download