Abstract
Background and Objectives
Subjects and Methods
Results
Acknowledgments
References
Fig. 1
Etiologies and aggravating factors of acute heart failure. (A) Etiologies of acute heart failure, (B) aggravating factors of acute heart failure, (C) aggravating factors in ischemic and non-ischemic cardiomyopathies. acute coronary syndrome. NSAIDs: non-steroidal anti-inflammatory drugs, ACS: acute coronary syndrome.
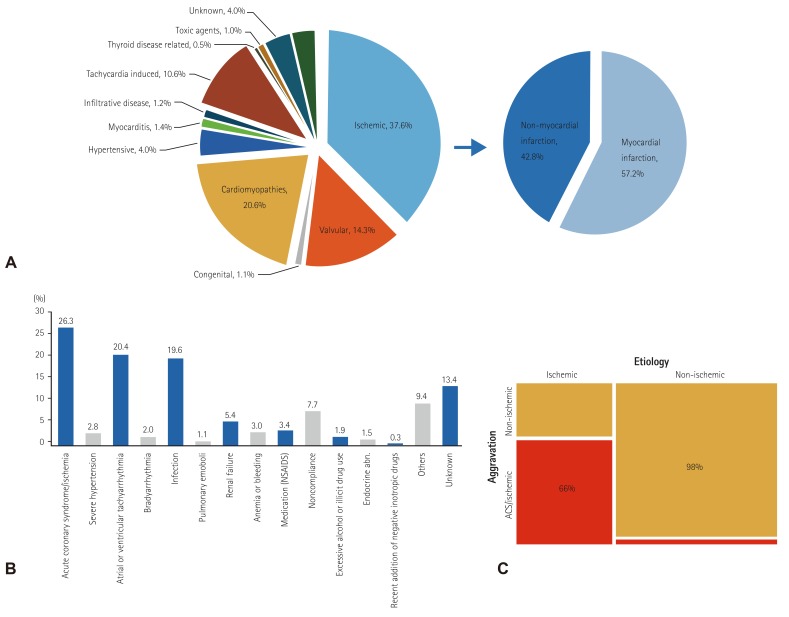
Fig. 2
Evidence-based medication prescriptions. (A) Prescription rate of angiotensin converting enzyme inhibitors: angiotensin receptor blockers: beta-blockers and aldosterone antagonists for acute heart failure patients. (B) Prescription rate changes in evidence-based medication before and after interim analysis. LVEF: left ventricular ejection fraction, ACEIs: angiotensin converting enzyme inhibitor, ARBs: angiotensin receptor blockers, BBs: beta-blockers, AAs: aldosterone antagonist.
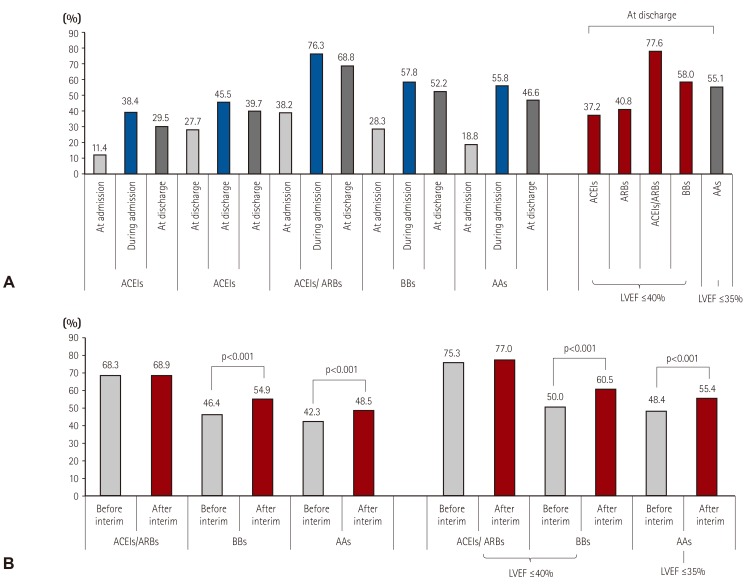
Fig. 3
Clinical outcomes of acute heart failure. (A) Mechanism of in-hospital mortality, (B) Kaplan-Meier survival curve for all-cause mortality (blue) and re-hospitalization due to heart failure aggravation (red) after discharge. HF: heart failure.
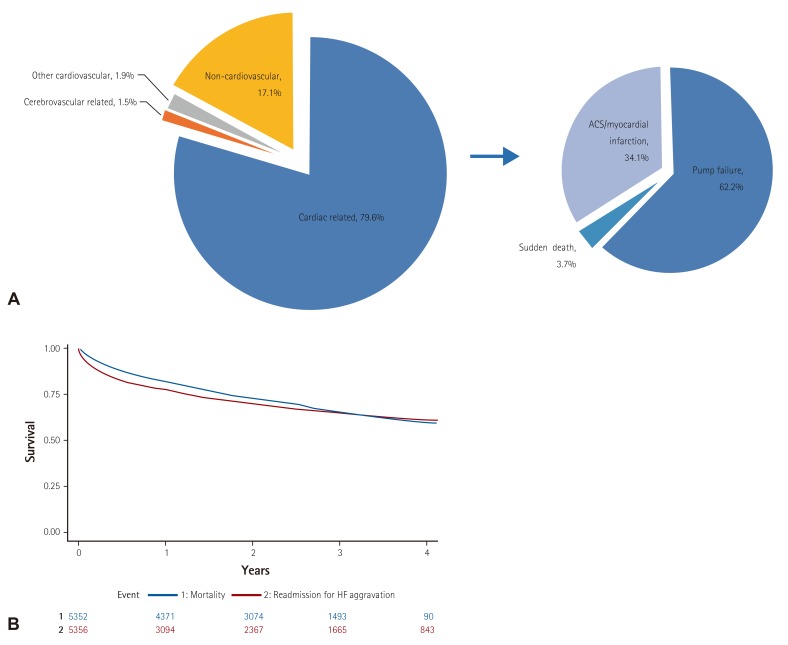
Table 1
Baseline characteristics, clinical management, and outcomes
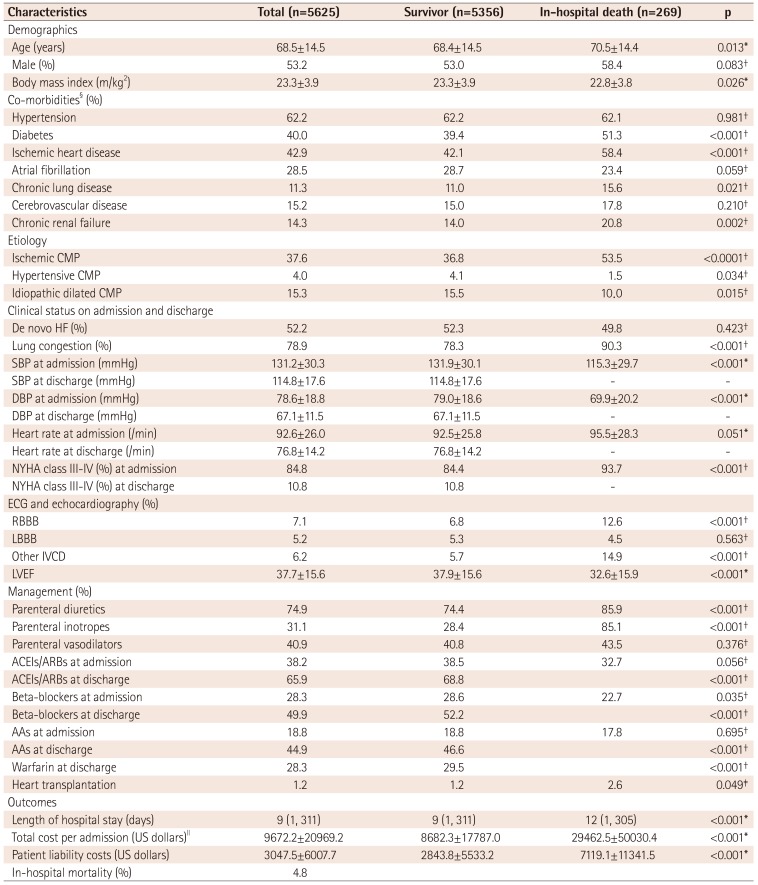
Values are presented as mean±standard deviation, median (min, max) or n (%). *A p value by Wilcoxon rank sum test, †p value by Chi-square test, ‡p value by Fisher's exact test, §includes in-hospital diagnoses, IIUS$ 1 is 1100 Korean won. CMP: cardiomyopathy, HF: heart failure, SBP: systolic blood pressure, DBP: diastolic blood pressure, ECG: electrocardiography, RBBB: right bundle branch block, LBBB: left bundle branch block, IVCD: intraventricular conduction delay, LVEF: left ventricular ejection fraction, ACEIs: angiotensin converting enzyme inhibitors, ARBs: angiotensin receptor blockers, AAs: aldosterone antagonists
Table 2
Laboratory tests on admission and discharge
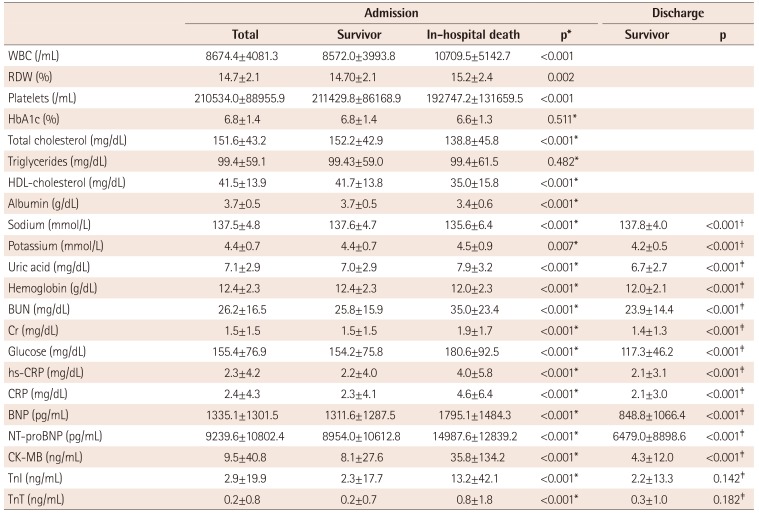
Values are presented as mean±standard deviation, median (min, max) or n (%). *A p value by Wilcoxon rank sum test between survivors and non-survivors, †p value by paired t-test between survivor levels at admission and discharge, ‡p value by Wilcoxon signed rank test between survivor levels at admission and discharge. WBC: white blood cells, RDW: red cell distribution width, HbA1c: hemoglobin A1c, HDL: high density lipoprotein, BUN: blood urea nitrogen, Cr: creatinine, hs-CRP: high sensitivity C-reactive protein, CRP: C-reactive protein, BNP: brain natriuretic peptides, NT-proBNP: N-terminal pro-brain natriuretic peptides, CK-MB: creatine kinase-MB, TnI: troponin I, TnT: troponin T
Table 3
Hospital treatment
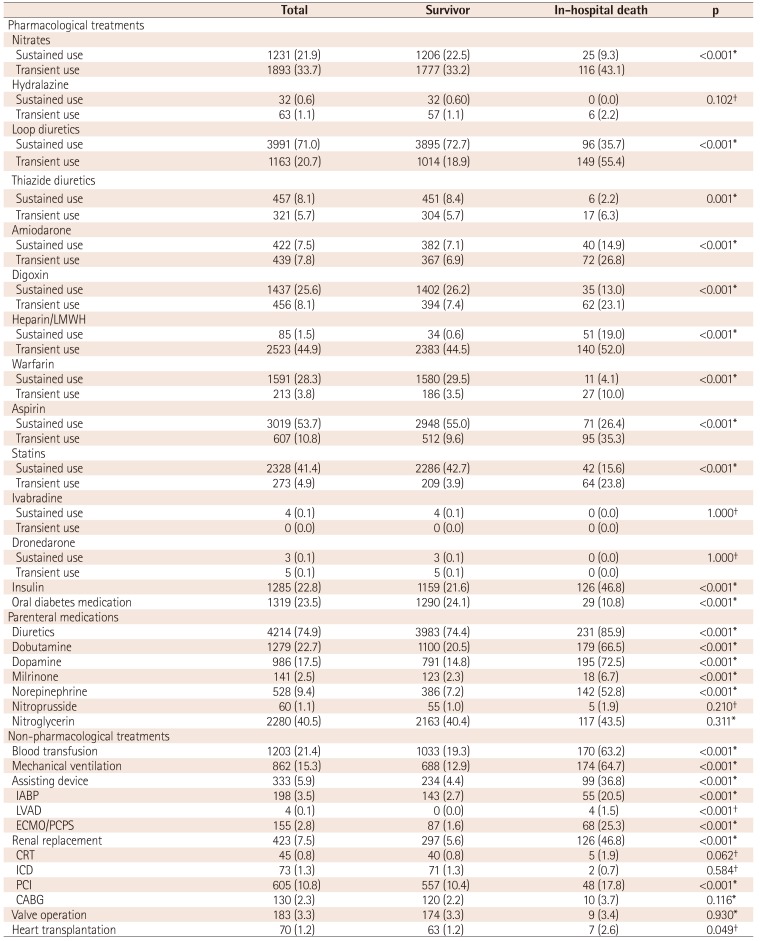
*A p value by Chi-square test, †p value by Fisher's exact test. LMWH: low-molecular weight heparin, IABP: intra-aortic balloon pump, LVAD: left ventricular assistant device, ECMO: extracorporeal membrane oxygenation, PCPS: percutaneous cardiopulmonary support, CRRT: continuous renal replacement therapy, CRT: cardiac resynchronization therapy, ICD: implantable cardioverter defibrillator, PCI: percutaneous coronary intervention, CABG. coronary artery bypass graft
Table 4
Multivariable logistic regression for in-hospital mortality and multinomial logistic regression for cardiovascular or non-cardiovascular death
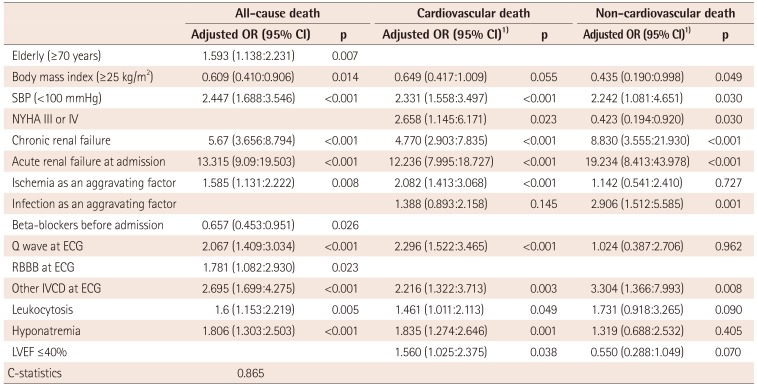
OR: odds ratio, CI: confidential interval, SBP: systolic blood pressure, ECG: electrocardiography, RBBB: right bundle branch block, IVCD: intraventricular conduction delay, Leukocytosis: white blood cell count ≥10000/mm3, Hyponatremia: serum sodium <135 mmEq/L, LVEF: left ventricular ejection fraction
Table 5
KorAHF and KorHF comparisons
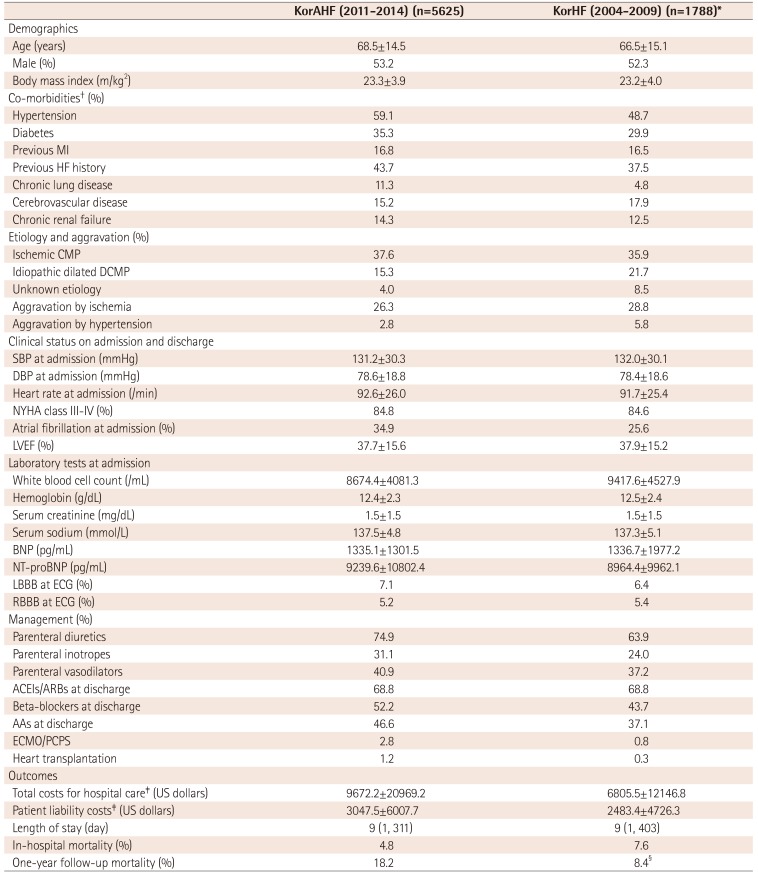
Values are presented as mean±standard deviation or number (%). *Population of hospitals participating KorAHF, †from past medical history, ‡US$ 1 is 1100 Korean won, §mortality data was not validated in all patients. KorAHF: Korean acute heart failure, KorHF: Korean heart failure, MI: myocardial infarction, HF: heart failure, CMP: cardiomyopathy, SBP: systolic blood pressure, DBP: diastolic blood pressure, LVEF: left ventricular ejection fraction, BNP: brain natriuretic peptides, NT-proBNP: N-terminal pro-brain natriuretic peptides, RBBB: right bundle branch block, LBBB: left bundle branch block, ECG: electrocardiography, ACEIs: angiotensin converting enzymes, ARBs: angiotensin receptor blockers, AAs: aldosterone antagonists, ECMO: extracorporeal membrane oxygenation, PCPS: percutaneous cardiopulmonary support




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download