Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
The change of exercise capacities in group 1 after PVR. Group 1: post PVR VO2peak were increased, as compared to pre PVR VO2peak. The mean VO2at improved from 24.15 to 25.38 mL/kg/min (p=0.051), the mean VO2peak from 28.53 to 33.09 mL/kg/min (p=0.001). PVR: pulmonary valve replacement, VO2at: anaerobic threshold, VO2peak: peak oxygen uptake.

Fig. 2
The change of exercise capacities in group 2 after PVR Group 2: post PVR VO2peak were decreased, as compared to pre PVR VO2peak. The mean VO2at decreased from 23.58 to 20.83 mL/kg/min (p=0.030), the mean VO2peak from 31.24 to 27.39 mL/kg/min (p<0.001). PVR: pulmonary valve replacement, VO2at: anaerobic threshold, VO2peak: peak oxygen uptake.

Fig. 3
The comparison of pre PVR predicted VO2peak (%) between the two groups. Group 1: post PVR VO2peak were increased, as compared to pre PVR VO2peak. Group 2: post PVR VO2peak were decreased, as compared to pre PVR VO2peak. Pre PVR Predicted VO2peak (%) value was significantly lower in patients belonging to group 1 (60.83±10.28) than group 2 (75.81±13.83) (p=0.003). PVR: pulmonary valve replacement, PredVO2peak (%): percentage of predicted VO2peak.

Fig. 4
The comparison of Δpredicted VO2peak (%) between the two groups. Group 1: post PVR VO2peak were increased, as compared to pre PVR VO2peak. Group 2: post PVR VO2peak were decreased, as compared to pre PVR VO2peak. The changes of predicted VO2peak (%) in patients belonging to group 1 were significantly higher than group 2 (10.17±7.63 vs. -10.56±8.03, p<0.001). PVR: pulmonary valve replacement, PredVO2peak (%): percentage of predicted VO2peak.

Fig. 5
The correlation between ΔRVEF (%) and ΔVO2at in patients belonging to group 1. Group 1: post PVR VO2peak were increased, as compared to pre PVR VO2peak. The change of RVEF (%) was positively correlated with the change of VO2at (r=0.733, p=0.007) in patients who showed increased VO2peak after PVR. RVEF: right ventricular ejection fraction. VO2at: anaerobic threshold, VO2peak: peak oxygen uptake, PVR: pulmonary valve replacement.

Fig. 6
The correlation between ΔRVEF (%) and Δpredicted VO2peak (%) in patients belonging to group 2. Group 2: post PVR VO2peak of patients were decreased, as compared to pre PVR VO2peak. The change of RVEF (%) was negatively correlated with the change of predicted VO2peak (%) (r=-0.575, p=0.020) in patients who showed decreased VO2peak after PVR. PVR: pulmonary valve replacement, RVEF: right ventricular ejection fraction. VO2peak: peak oxygen uptake. PredVO2peak (%): percentage of predicted VO2peak.

Table 1
Patient demographic data (n=28)
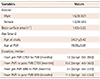
Table 2
The changes of CPEX and MRI parameters after PVR
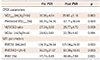
Values are presented as mean±standard deviation or number (%). CPEX: cardiopulmonary exercise, MRI: magnetic resonance imaging, PVR: pulmonary valve replacement, VO2peak: peak oxygen uptake, predicted VO2peak (%): percentage of predicted VO2peak, VE/VCO2: slope of respiratory minute volume to CO2 production, VO2at: anaerobic threshold, RV EDVI: right ventricular end diastolic volume index, RV ESVI: right ventricular end systolic volume index, RVEF: right ventricular ejection fraction
Table 3
Baseline characteristics of the two groups

Values are presented as mean±standard deviation or number (%). Group 1: post PVR VO2peak of patients were increased compared to pre PVR VO2peak. Group 2: post PVR VO2peak of patients were decreased compared to pre PVR VO2peak. CPEX: cardiopulmonary exercise, PVR: pulmonary valve replacement, MRI: magnetic resonance imaging
Table 4
The comparison of CPEX parameters between the two groups

Values are presented as mean±standard deviation or number (%). Group 1: post PVR VO2peak of patients were increased compared to pre PVR VO2peak. Group 2: post PVR VO2peak of patients were decreased compared to pre PVR VO2peak. CPEX: cardiopulmonary exercise, PVR: pulmonary valve replacement, VO2peak: peak oxygen uptake, predicted VO2peak (%): percentage of predicted VO2peak, VE/VCO2: slope of respiratory minute volume to CO2 production, VO2at: anaerobic threshold
Table 5
The comparison of MRI parameters between the two groups

Values are presented as mean±standard deviation or number (%). Group 1: post PVR VO2peak of patients were increased compared to pre PVR VO2peak. Group 2: post PVR VO2peak of patients were decreased compared to pre PVR VO2peak. MRI: magnetic resonance imaging, PVR: pulmonary valve replacement, RV EDVI: right ventricular end diastolic volume index, RV ESVI: right ventricular end systolic volume index, RVEF: right ventricular ejection fraction
Table 6
The correlation between RVEF (%) and CPEX parameters in each group

Group 1: post PVR VO2peak of patients were increased compared to pre PVR VO2peak. Group 2: post PVR VO2peak of patients were decreased compared to pre PVR VO2peak. CPEX: cardio pulmonary exercise, r: correlation coefficient, VO2peak: peak oxygen uptake, Predicted VO2peak (%): percentage of predicted VO2peak, VE/VCO2: slope of respiratory minute volume to CO2 production, VO2at: anaerobic threshold, RVEF: right ventricular ejection fraction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download