Abstract
Acute malperfusion syndrome is a serious complication of acute aortic dissection. A 76-year-old female patient was admitted with acute type B aortic dissection and developed renal malperfusion during medical therapy. We are reporting a clinically successful result from the thoracic endovascular aortic repair used for malperfusion syndrome that occurred by acute type B aortic dissection.
Medical therapy remains the standard of care in the treatment of acute type B aortic dissection, despite its considerable long term complications. Since thoracic endovascular aortic repair (TEVAR) was introduced as an alternative treatment option for patients with type B aortic dissection in 1999, the use of TEVAR has been increasing in acute type B aortic dissection. However, acute complicated type B aortic dissections with distal malperfusion are a catastrophic process in association with life-threatening ischemia of the viscera and extremities. We are reporting a clinically successful result from the TEVAR used for malperfusion syndrome that occurred by acute type B aortic dissection.
A 76-year-old female was admitted to the emergency room of our hospital with the sudden onset of upper abdominal and back pain. At the time of presentation, blood pressure, heart rate, and O2 saturation were 133/70 mm Hg, 70 beats/minute, and 95%, respectively.
An emergent chest computed tomography was performed under the suspicion of an acute aortic dissection, which showed an acute stanford type B aortic dissection. The patient was then transferred to intensive care unit for strenuous supportive care. During the first 2 days of anti-impulse therapy and intensive care, abdominal and back pain improved. She was then transferred to the general ward.
However, on the 8th day, she complained of general edema and indigestion, while her blood creatinine level was elevated from 0.6 mg/dL to 2.9 mg/dL. A follow-up chest CT revealed that the size of false lumen in descending aorta was increased and that of true lumen decreased significantly. We concluded that increased blood flow in false lumen significantly decreased the rate of blood flow in branched vessels of the descending aorta, which then developed malperfusion syndrome (Fig. 1).
We decided that emergent operation was very risky because of the patient's old age, poor nutritional state and acute renal failure. Therefore, the TEVAR was selected as an alternative. The primary goal of TEVAR was to cover the primary tearing site in descending aorta around the diaphragm to reduce the flow of the false lumen and expand the true lumen. The patient was transferred to cardiac intervention room. The patient then underwent general anesthesia, and the right common femoral artery was exposed. The guiding wire and diagnostic catheter was introduced through the right femoral artery. After the locating the true lumen and the primary tearing site, 30×26×150 mm SEAL aortic stent graft (S&G Biotech, Seongnam, Korea) was introduced through the right femoral artery. The primary tear at the level of distal thoracic aorta was covered by the stent graft, and the distal end of stent graft was then deployed proximal to the celiac axis. After the stent graft insertion was completed, the aortogram showed an increased flow of the true lumen and the perfusion of the branch vessels in the abdominal aorta was recovered (Fig. 2).
After TEVAR, the general edema, abdominal and back pain subsequently resolved, and the blood creatinine level went down to 1.3 mg/dL. On the 8th day after TEVAR, she was discharged without any procedure-related complications. During the 1 year clinical follow-up, the patient had remained stable and had no evidence of a cardiovascular event.
For patients with acute uncomplicated type B aortic dissection, aggressive medical management with anti-implusive therapy has been used and in-hospital survival rate approaches 90% with medical therapy alone.1)
However, the complications of branch vessels are developed in 30-50% of aortic dissection, and the mortality of complicated aortic dissection is at least, 60%.2) Conventional therapy in acute complicated type B aortic dissections is open repair surgery of the aorta, via a resection of the primary tear site. However, results with operative therapy for acute complicated type B aortic dissections have been disappointing, which shows an early mortality rate approaching 40%.2-4)
Because the morbidity and mortality associated with conventional open repair is very high, endovascular therapy have emerged as the alternative for the management of acute complicated type B aortic dissection. Also, patients with acute complicated type B aortic dissections with malperfusion syndrome have remained as a challenging group to manage.
The natural course of acute type B aortic dissection depends on the patency of the false lumen. During the follow-up, if the false lumen in descending aorta was filled with thrombus, the probability of developing an aortic aneurysm would decrease. However, if the patent flow of false lumen existed, there would be a greater chance of developing an aortic aneurysm and it could disturb the flow of branch vessels, causing ischemic injury to multiple organs.
Mechanisms of the malperfusion syndrome have been well described by Williams et al.3)4) The pathophysiology of malperfusion in acute complicated type B aortic dissections is composed of both dynamic and static obstruction of the involved branch vessel. The intimal flap in dynamic obstruction can intermittently cause occlusion of the branch vessel origin during the cardiac cycle, and this subsequently, results in end-organ ischemia. In static obstruction, the dissection flap is progressed to the branch vessel origin and causes end organ ischemia by disturbing the flow of true lumen.
The percutaneous fenestration and TEVAR have been used in case of flow disturbance of branch vessels. The percutaneous fenestration is superior in a sense that the flow of the true lumen can be recovered through the fenestration, but the patent false lumen can still exist and it can be aggravated and cause aortic aneurysm.5) The primary goal of endovascular treatment is to cover the primary tear site, thus, increasing the chance of expanding the true lumen, while obliterating the false lumen and the thrombus formation. Many studies examining the role of TEVAR in acute complicated type B aortic dissection have been encouraging.6)7) Therefore TEVAR has been increasingly utilized as an alternative to conventional open repair.
In our hospital, we're reporting successful results of the TEVAR to patients having the malperfusion syndrome caused by acute type B aortic dissection.
Figures and Tables
Fig. 1
Preoperative chest CT scans. A: the initial CT scan shows acute type B aortic dissection. B: follow-up chest CT scan shows that the size of true lumen of celiac artery and renal artery have been decreased significantly due to increasing false lumen pressure in descending aorta (the arrow indicates false lumen flow).
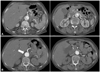
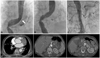
Fig. 2
Periperative aortograms and follow-up chest CT scans. A: the arrow indicates suspicious tearing site in aortogram. B: aortogram after thoracic endovascular aortic repair. Stent graft was positioned from distal thoracic aorta to just above celiac axis. C: restoration of blood flow in the abdominal branches after stent grafting. D: the postoperative CT scan shows that the size of true lumen in descending aorta have been increased and false lumen successfully have been obliterating.
References
1. Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006. 114:2226–2231.
2. Lauterbach SR, Cambria RP, Brewster DC, et al. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg. 2001. 33:1185–1192.
3. Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: part III. anatomy and radiologic diagnosis of branch-vessel compromise. Radiology. 1997. 203:37–44.
4. Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications--principles and results. J Vasc Interv Radiol. 1997. 8:605–625.
5. Panneton JM, Teh SH, Cherry KJ Jr, et al. Aortic fenestration for acute or chronic aortic dissection: an uncommon but effective procedure. J Vasc Surg. 2000. 32:711–721.
6. Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation. 2003. 108:Suppl 1. II312–II317.
7. Chen S, Yei F, Zhou L, et al. Endovascular stent-grafts treatment in acute aortic dissection (type B): clinical outcomes during early, late, or chronic phases. Catheter Cardiovasc Interv. 2006. 68:319–325.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download