Abstract
Most type I and II perforations are predominately caused by hydrophilic and stiff wires, often presented in the delayed form, and do not require pericardial drainage or surgical interventions. However, we report a type III delayed coronary artery perforation at the site of stent implantation after intervention without any evidence of immediate perforations. To the best of our knowledge, this is the first case report of angiographic documentation and treatment of delayed coronary perforation at the site of stent, presented as a cardiac arrest.
Coronary artery perforation (CP) during percutaneous coronary intervention (PCI) is a rare complication. The reported incidence of CPs ranges from 0.1% to 0.6%.1-7) Most CPs occur during the procedure, immediately after stenting or adjunctive ballooning. However, it is also well recognized that the hydrophilic wires and glycoprotein IIb/IIIa antagonist during PCI increases the risk of delayed CP.2) However, those CP seemed to be minor and do not require pericardial drainage. However, we herein describe an unusual delayed CP (type III) at the site of stent implantation after PCI without any evidence of immediate perforations. To the best of our knowledge, this is the first case report of angiographic documentation and treatment of delayed CP at the site of stent, presented as cardiac arrest.
A 64 year-old male patient was admitted for chest pain. He had no specific cardiovascular risk factors except for a history of heavy smoking. Coronary angiogram (CAG) showed critical stenosis of the left circumflex artery (Fig. 1A).
Percutaneous coronary intervention was performed with the use of 6 Fr guiding catheter and hydrophilic guide-wire via trans-radial sheath. After wiring, pre-dilatation was done with a 2.0×15 mm coronary balloon. Next, an Endeavor splinter™ (3.5×18 mm) was implanted with 10 atmosphere. After stenting, intravascular ultrasound (IVUS) showed suboptimal expansions (Fig. 1B). Therefore, adjunctive ballooning was performed with a 3.75×10 mm noncompliant balloon catheter. Final the CAG showed no residual stenosis without any complications and final IVUS images revealed good apposition of the stent without any abnormal findings (Fig. 1C). Finally, we finished the procedure and the patient was transferred to the general ward. Till then, it was a routine PCI and no problems developed.
However, the patient complained of severe chest pain immediately after being moved to a general ward. Cardiac arrest developed just at the moment of chest pain. The initial monitoring showed flat electrocardiography. Therefore, cardiovascular resuscitation (CPR) was performed for 15 minutes, but there was no response and the pulse rate was not recovered. Retrospective analysis of the final angiogram, however, led to the suspicion that acute stent thrombosis of drug-eluting stent or a delayed dissection of the major coronary artery may be the cause of cardiac arrest at that time. Therefore, we decided to perform a CAG again during CPR.
Before angiography, percutaneous cardiopulmonary support was immediately applied via the femoral artery during CPR. Next, the CAG was checked again. Surprisingly, CAG revealed coronary perforation with active bleeding (type III) at the previous stent implantation site (Fig. 2A). Pericardiocentesis was performed successfully and Jo graft stent (3.0×19 mm) was implanted for the active bleeding site (Fig. 2B). However, the leakage of blood still remained. Therefore, we considered another graft stent. However, while preparing for another graft stent, the cardiac arrest redeveloped. The CPR was restarted and we were able to determine what the second problem was. Tension pneumothorax developed during ambu bagging, so we decided to insert the chest tube. Therefore, after insertion of another graft stent (3.5×19 mm), the chest tube was inserted to the pleural cavity by a thoracic surgeon simultaneously. After that, the vital signs recovered and bleeding was controlled (Fig. 2C). Finally, we were able to finish the procedures. The pneumothorax was resolved after 3 days and cardiac function totally recovered within 3 days. However, the brain of the patient was damaged by hypoxic brain injury and he remained in a vegetative state.
Coronary artery perforation was defined as evidence of extravasation of contrast dye or blood from the coronary artery during or following a coronary interventional procedure. Recently, the incidence of CP has increased slightly. Possibly due to various PCI devices being developed and more complex lesions or smaller vessels being included as the target lesions for PCI. The development of debulking devices has enabled the treatment of calcified and bifurcation lesions. The perforation type was determined based on the Ellis classification.1) A classification was based on angiographic appearance of the perforation (I, extraluminal crater without extravasation; II, pericardial or myocardial blushing without contrast jet extravasation; III, extravasation though frank perforation with ≥1-mm diameter). The outcomes of CP varied considerably with the grade of perforation. Patients who sustained type I or II CP showed a tendency of good prognoses but, in contrast, patients with type III perforations had a higher mortality rate.
According to a previous report,2) pericardial effusion or tamponade rarely became clinically manifested after several hours following the coronary procedure even though vessel perforation was not clinically or angiographically obvious at the end of the procedure. Also, the mechanism of vessel perforation was believed to be a distal branch puncture with the guidewire tip such as a hydrophilic wire and the administration of glycoprotein IIb/IIIa antagonist. However, most of the type I and II perforations are predominately caused by hydrophilic and stiff wires, and do not require pericardial drainage or surgical intervention.8) Type III perforations are more often associated with stent and device use, and manifested immediately. A majority of type III perforations can be initially managed by percutaneous methods.
In this case, we have shown a very rare possibility which could have been a neglected cause of a sudden cardiac arrest after PCI. In most cases of sudden arrest within 24 hours after PCI, and especially when the time of death, the causes of death are almost always assumed to be cardiac arrest by a stent thrombosis or by malignant arrhythmia (ex. ventricular tachycardia) unless the patient has an autopsy. For the diagnosis of CP after PCI, echocardiography was the preferred method, and pericardial fluid collection on echocardiography could be a clue for assuming CP after PCI. Definite diagnosis was made by confirming the extravasation of contrast dye or blood in coronary angiography. However, in many cases, even CP strongly assumed by echocardiography, coronary angiography could not find the exact focus of perforation.
An interesting point of the present case is that the type III perforations occurred late after the procedures and presented as a cardiac arrest. If we did not confirm CP angiographically, we could not have known the cause of cardiac arrest and it was reasonable to assume that the cause may be a probable acute stent thrombosis even when there was no evidence of it.
The mechanism of delayed type III perforations was unclear. We could not detect any evidence of coronary artery rupture in the final angiogram and IVUS study. However, we may postulate heavy coronary calcification and use a larger adjunctive coronary balloon.
In conclusion, this case highlights that delayed CP exists in clinical practice and could be misdiagnosed as other events such as an acute stent thrombosis or malignant ventricular arrhythmia. Sudden deterioration of a patient after PCI with a high risk of perforation such as tortuous calcified lesions, CP should be considered as one of the causes of deterioration.
Figures and Tables
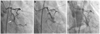
Fig. 1
Coronary angiogram for pecutaneous coronary intervention for critical stenosis of left. Circumflex artery. A: coronary angiogram showed critical stenosis of left circumflex artery (arrow). B: after stenting, angiogram showed mild residual stenosis in proximal stented lesion and intravascular ultrasound showed incomplete expansions. C: final angiogram showed no residual lesion and final intravascular ultrasound images revealed improved expansion and good apposition of the stent.

Fig. 2
Coronary angiogram after cardiovascular resuscitation due to sudden cardiac arrest in general ward. A: angiogram showed type III coronary perforation with bleeding at the previous stent implantation site (arrow). B: before inflation, first Jo graft stent was placed for the active bleeding site. C: after stenting of two graft stents, the final angiogram during cardiovascular resuscitation showed no additional bleeding.
References
1. Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era: incidence, classification, management, and outcome. Circulation. 1994. 90:2725–2730.
2. Gunning MG, Williams IL, Jewitt DE, Shah AM, Wainwright RJ, Thomas MR. Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart. 2002. 88:495–498.
3. Gruberg L, Pinnow E, Flood R, et al. Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2000. 86:680–682.
4. Shimony A, Zahger D, Van Straten M, et al. Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2009. 104:1674–1677.
5. Von Sohsten R, Kopistansky C, Cohen M, Kussmaul WG 3rd. Cardiac tamponade in the "new device" era: evaluation of 6999 consecutive percutaneous coronary interventions. Am Heart J. 2000. 140:279–283.
6. Fasseas P, Orford JL, Panetta CJ, et al. Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J. 2004. 147:140–145.
7. Fukutomi T, Suzuki T, Popma JJ, et al. Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J. 2002. 66:349–356.
8. Ramana RK, Arab D, Joyal D, et al. Coronary artery perforation during percutaneous coronary intervention: incidence and outcomes in the new interventional era. J Invasive Cardiol. 2005. 17:603–605.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download