Abstract
Infective endocarditis is a life-threatening condition caused by microbial infection of the heart's endocardial surface. This condition can also be associated with bacterial infections of other organs. We experienced an unusual case of recurrent infective endocarditis associated with pyogenic spondylodiskitis. A 70-year-old man presented with persistent fever and lower back pain visited our hospital. The patient had a past history of recurrent infective endocarditis. He was diagnosed with infective endocarditis again based on clinical symptoms and echocardiographic findings. Magnetic resonance imaging was used to evaluate lower back pain, which showed acute spondylodiskitis on L3 and L4 vertebrae. The patient completely recovered following four weeks of antibiotic therapy.
Infective endocarditis is a life-threatening condition caused by microbial infection of the heart's endocardial surface. It most commonly involves the heart valves, but other sites including septal defects, chordae tendinae, and mural endocardium can be involved.
Common symptoms of infective endocarditis include fever, anorexia, weight loss, malaise, night sweats, skin lesions (splinter hemorrhage, Osler's nodes, Janeway's lesions), conjunctival petechiae, and splenomegaly.
Occasionally, serious complications such as septic aneurysms and septic emboli can occur in major organs (kidney, brain, liver, spleen) which may result in death. Septic emboli may occur in the spine, but the incidence is extremely low.
We herein present our experience of a patient with infective endocarditis associated with pyogenic spondylodiskitis who was successfully treated with antimicrobial treatment.
A 70-year-old man presented to our hospital with a two-week history of fever associated with lower back pain. He had a past history of recurrent infective endocarditis, for which he was admitted to our hospital in 2005 and in 2009. Streptococcus bovis was isolated during the first visit in 2005 as the causative organism of infective endocarditis. In addition, vegetation was found on the posterior leaflet of the mitral valve. During the second admission, blood culture showed the growth of a Streptococcus species. However, specific culture results were not reported. Similar to the first admission, vegetation growth was again found on the posterior leaflet of the mitral valve. On both admissions, the patient successfully recovered following the appropriate antibiotic treatment with reduction of vegetation.
On admission, the patient's core body temperature was 39.0℃, blood pressure 130/80 mmHg, and pulse rate was 88 beats/minute. Auscultation revealed a regular heart beat with an early systolic murmur was found at the left lower sternal border (grade III/VI). Neurological examination was unremarkable. No peripheral stigmata of infective endocarditis were noted.
Laboratory tests showed absence of leukocytosis (white blood cell 7,400/mm3), but mild anemia (hemoglobin 8.5 mg/dL), and thrombocytopenia (platelets 120,000/mm3) was noted. Except for a C-reactive protein (CRP) value of 4.17 mg/dL (0.1-0.8 mg/dL), no other laboratory tests showed significant abnormalities (blood urea nitrogen 15 mg/dL, creatinine 0.8 mg/dL, total protein 6.9 g/dL, albumin 3.4 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 14 IU/L).
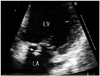
Chest radiography showed no pulmonary edema or active lesions in the lungs. In transthoracic echocardiography, a 2.7×1.4 cm sized vegetation on the posterior leaflet of the mitral valve (Fig. 1) was noted. Furthermore, moderately severe mitral regurgitation, trivial aortic regurgitation, and moderate tricuspid regurgitation was also noted. Other findings included left ventricular (LV) hypertrophy (LV mass 270.5 gm), LV enlargement (LV end diastolic dimension 61 mm, LV end systolic dimension 41 mm), left atrial (LA) enlargement (LA dimension size 40.1 mm), and mild pulmonary hypertension (pulmonary arterial pressure 37 mmHg).
After diagnosis of infective endocarditis was confirmed, treatment was immediately started with intravenous ceftriaxone and gentamycin, on suspicion of recurrent infective endocarditis.
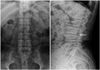
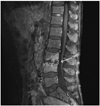
On the second day of antibiotic therapy, fever and other signs of infection resolved. We continued antibiotic treatment for four weeks. However, blood culture demonstrated no growth of bacteria and fungi. Although his clinical signs and symptoms related to infective endocarditis improved, and CRP level decreased, his back pain did not completely resolve. Consequently, we decided to order a plain lumbar spine radiograph to evaluate the lower back pain, which showed disc space narrowing and endplate erosion of the L3 and L4 vertebral bodies (Fig. 2). Magnetic resonance imaging of the lumbar spine was performed to further evaluate the erosive lesions, which confirmed acute spondylodiskitis of L3, L4 vertebrae and L3-4 disc space (Fig. 3). Pyogenic spondylodiskitis was highly suspicious to be associated with infective endocarditis according to the clinical course and patient's history. The patient was discharged after four weeks of intravenous antibiotic treatment without complication. We decided to conduct follow-up evaluation of the patient's spinal lesions at the orthopedic outpatient clinic.
During follow-up at the outpatient clinic, the size of mitral valve vegetation was shown to be reduced on echocardiography (1.35×0.67 cm). There were no symptoms of infective endocarditis or pyogenic spondylodiskitis.
Infectious diskitis (spondylodiskitis, spondylodiscitis, infectious spondylitis) is an inflammatory process that involves one or more extradural components of the spine.1) Although it affects a small proportion (2-7%) of all patients with osteomyelitis, it has clinical importance due to potential morbidity and mortality. Spondylodiskitis commonly involves the lumbar (45%), thoracic (35%), and cervical (10-20%) spines.2) Secondary epidural abscess formation occurs most frequently in the cervical spine, followed in frequency by the thoracic and lumbar spine.2)
Although the clinical presentation of patients with spondylodiskitis varies, it generally commences with insidious development of localized back pain combined with non-specific symptoms, such as malaise, fever, and weight loss. Our patient also experienced these symptoms. As fever and leukocytosis are often absent, symptoms of spondylodiskitis may be present for months before the diagnosis is confirmed. This may result in progression of the local disease process. Patients may present with hip contracture or paralysis secondary to abscess formation in the paraspinal or epidural spaces.
Pyogenic spinal infections are most commonly caused by Staphylococcus aureus (in 60% of all patients) and Enterobacter species (in 30% of all patients).1)3)4) Pseudomonas aeruginosa, Serratia species, and Candida species most often affect patients with a history of intravenous drug abuse. Mycobacterium tuberculosis causes most non-pyogenic spinal infections. However, fungi (e.g., Cryptococcus species, Aspergillus species, coccidioidomycosis) also may cause infections.5-8) In spondylodiskitis, the three main routes of infection are hematogenous spread, direct inoculation, and contiguous spread. In adults, most cases result from direct inoculation after spinal instrumentation procedures, including surgery, discography, and epidural injections. Spontaneous infections result from a hematogenous source (e.g., bacteremia, intravenous drug abuse) usually beginning at a lumbar or thoracic vertebral body subjacent to the vertebral endplate. Loss of disk height may occur as pyogenic organisms release enzymes that dissolve the nucleus pulposus. Non-pyogenic organisms, such as tuberculosis do not produce proteolytic enzymes. Therefore, they tend to spare the disk from destruction. Spread from an adjacent source, such as a psoas abscess, is an uncommon mechanism. CSF and lymphatic spread are also uncommon routes of infection.9)
In our patient, pyogenic spondylodiskitis was associated with recurrent infective endocarditis. Several cases of infective endocarditis associated with spondylodiskitis have been reported, however to the best of our knowledge, there was no reported case in Korea.10)11) Pigrau et al.12) investigated the incidence and risk factors of infective endocarditis in patients with pyogenic vertebral osteomyelitis. A retrospective record review was conducted on all cases of vertebral osteomyelitis from January 1986 to June 2002 that occurred at a tertiary referral hospital. Among 606 patients with infective endocarditis, 28 cases (4.6%) had pyogenic vertebral osteomyelitis. Among 91 cases of pyogenic vertebral osteomyelitis, 28 cases (30.8%) were associated with infective endocarditis. In 6 patients, there were no clinical signs of infective endocarditis, and the diagnosis was established only by routine echocardiography. When specifically investigated, the incidence of infective endocarditis is high in patients with pyogenic vertebral osteomyelitis. Therefore, in pyogenic vertebral osteomyelitis patients, routine echocardiography is valuable for early detection of infective endocarditis.
Figures and Tables
References
1. Hopkinson N, Stevenson J, Benjamin S. A case ascertainment study of septic discitis: clinical, microbiological and radiological features. QJM. 2001. 94:465–470.
2. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008. 56:401–412.
3. Ponte CD, McDonald M. Septic discitis resulting from Escherichia coli urosepsis. J Fam Pract. 1992. 34:767–771.
4. Park CB, Kim JJ, Song JK, et al. Right-Sided Infective Endocarditis in Korea. Korean Circ J. 2005. 35:633–638.
5. Moreillon P, Que YA. Infective endocarditis. Lancet. 2004. 363:139–149.
6. Duval X, Leport C. Prophylaxis of infective endocarditis: current tendencies, continuing controversies. Lancet Infect Dis. 2008. 8:225–232.
7. Kim MK, Song JK, Kang DH, et al. Recent trends and clinical outcomes of infective endocarditis. Korean J Med. 2000. 58:28–38.
8. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007. 116:1736–1754.
9. Yi MZ, Lee SH, Park CB, et al. Clinical characteristics of nosocomial infective endocarditis in a tertiary referral hospital. Korean Circ J. 2006. 36:236–241.
10. Talsania N, Ogundipe O. Infective discitis mimicking infective endocarditis and osteoarthritic back pain. Internet J Rheumatol. 2005. 2:3.
11. Udayaraj UP, Gendi NS, Osman EM. Septic discitis as a complication of infective endocarditis caused by Streptococcus oralis. J Rheumatol. 2003. 30:632–633.
12. Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005. 118:1287.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download