Abstract
Neointimal hyperplasia mainly develops within several months of coronary stent deployment, after which it stabilizes. Although it was widely accepted, particularly during the bare-metal stent (BMS) era, that in-stent restenosis (ISR) generally does not present as an acute coronary syndrome (ACS), but rather as a gradual recurrence of angina symptoms, recent data have shown that a substantial number of patients with ISR present as ACS. There has also been consistent postmortem evidence of plaque rupture secondary to atherosclerotic change within the neointima of a BMS. We report here a case of ACS in which intravascular ultrasound and optical coherent tomographic assessments revealed neointimal atherosclerotic change and ruptured plaque 10 years after BMS deployment.
Several authors recently reported a substantial number of bare-metal stent (BMS)-related restenosis cases presenting as acute coronary syndromes (ACS)1)2) and several case reports providing intravascular ultrasound (IVUS) evidence of pla-que rupture at in-stent neointima.3)4) Although IVUS is widely used to evaluate plaque composition, it has inherent image qu-ality limitations when compared with optical coherence tomography (OCT) for the assessment of soft tissue components and subtle surface changes in atherosclerotic lesions.5)6) Here, we present a patient with ACS in whom a combination of IVUS and OCT imaging showed atheromatous changes and plaque rupture of neointima 10 years after BMS implantation.
A 58-year-old man was admitted with unstable angina and complaining of new onset substernal chest pain at rest. The patient had a history of prior percutaneous coronary intervention (PCI) at this hospital due to angina pectoris ten years ago. At that time, the coronary angiogram (CAG) showed 90% stenosis at the proximal portion of the left anterior descending coronary (LAD). A BMS (NIR™, 3.75×16 mm, Medinol, Boston Scientific, Tel Aviv, Israel) was implanted at the proximal-portion of the LAD and there was no evidence of in stent restenosis (ISR) upon follow-up CAG nine months after index PCI. During ten years of follow-up at the local clinic, he had not complained of chest pain while on his medical treatment; however, periods of substernal chest pain at rest had recurred from one month before admission.
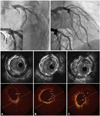
CAG on this admission revealed evidence of focal stenosis with luminal haziness on the previously stented proximal LAD and a de novo lesion at the distal left circumflex (LCX) artery (Fig. 1, upper panel). Upon quantitative coronary analysis, the previously stented lesion had a diameter stenosis of 65%. IVUS images demonstrated a well expanded previously deployed BMS and remarkable neointimal proliferation causing luminal narrowing (minimal luminal area=3.32 mm2, plaque burden=75.9%). Of interest was a suspicious cavitary-shaped ruptured plaque in the neointima (Fig. 1, middle panel). For more detailed evaluation of the suggested ruptured plaque in IVUS, we conducted OCT at the ISR site, which showed evident proximally located thin fibrous cap atheroma (TFCA) with lipid core and a ruptured plaque with tiny multiple ulcerations of neointima that were proximally located (Fig. 1, lower panel). A new-generation drug eluting stent (Xience V™, 4.0×28 mm, Abbott, IL, USA) was successfully placed to cover the previously stented lesion, and post-stenting IVUS revealed a well expanded previous ISR lesion (minimal stent area=7.31 mm2). A 2.75×23 mm stent was also successfully deployed in the de novo LCX lesion. At the nine months follow up, CAG showed patent stented lesions without evidence of ISR, and the patient has been clinically stable for 18 months after PCI.
Although many cardiologists are under the impression that neointimal growth after BMS implantation has benign consequences, this case shows unstable clinical features and consistent neointimal atheromatous changes and plaque rupture findings on IVUS and OCT.
In the past, post-mortem and endarterectomy specimens had shown extensive extracellular matrix accumulation with low rates of cellular proliferation in BMS-related ISR lesions,7-9) which were believed to have benign clinical presentation as stable angina.
Although awareness of very late stent thrombosis is usually associated with the drug-eluting stent (DES) era, it is also pertinent to the BMS era. A large retrospective study reported that the cumulative incidence of stent thrombosis after BMS implantation was 0.5% at 30 days, 0.8% at 1 year, 1.3% at 5 years, and 2.0% at 10 years.2) Another retrospective study of 1,186 cases of BMS-related ISR reported that more than one-third presented as acute myocardial infarction or unstable angina.1) These studies suggested that stent thrombosis and ACS long after BMS implantation might be attributable to neointimal plaque rupture. Recently, an interesting report on assessment of very late stent thrombosis after both DES and BMS using IVUS demonstrated that neointimal atherosclerotic changes and plaque ruptures were the main cause of BMS-related very late stent thrombosis.10) Another postmortem pathologic study revealed that atherosclerotic changes of neointima that occur significantly earlier and more frequently with DES, were also found with BMS.11)
Despite several previous reports suggesting atheromatous changes in neointima after stenting and case reports of IVUS-documented ruptured plaque in neointima, there have been few reports of atherosclerotic progression with ruptured plaques of in-stent intima confirmed by both IVUS and OCT. Because of its high resolution of approximately 10-20 µm, which is approximately 10-fold greater than that of IVUS, OCT can be a valuable tool for evaluation of intravascular plaque characterization that cannot be detected by CAG and IVUS.5)
In this case, we found a suspicious plaque rupture in the neointima using IVUS. However, OCT imaging allowed detection of more detailed atheromatous changes of neointima such as multiple tiny ulcerations and a TCFA with a large lipid core, which could not be precisely discriminated in IVUS images. OCT has a higher sensitivity than IVUS for characterizing lipid-rich plaques, and the higher resolution of OCT allows improved visualization of soft tissue components and detection of the cause of ACS such as plaque rupture or erosion when compared with IVUS images.5)6) We anticipate that neointimal atherosclerotic changes might be more easily detected before plaque rupture using a combination of OCT and IVUS than IVUS alone.
In summary, this case provides in vivo evidence of atheromatous neointimal changes and ruptured plaque using combination of IVUS and OCT imaging modalities.
Figures and Tables
Fig. 1
Coronary angiogram (upper panel), intravascular ultrasound (middle panel) and optical coherence tomography (lower panel) images of the in-stent restenosis site in the proximal left anterior descending artery. A: in comparisons of IVUS and OCT images in the corresponding site, an atherosclerotic plaque extending from 12 to 5 o'clock contains regions consistent with fibrous tissue, and a homogenous signal-poor lesion possible representing lipid core with thin cap fibrous atheroma (TFCA) was clearly shown in OCT (arrow). Although this fibrous atheromatous lesion was also apparent in the IVUS image from the same site, it was difficult to identify the presence of TFCA and lipid core. The minimal cap thickness at the region measured 60 m by OCT. B: immediately proximal to the ruptured plaque, the OCT image showed multiple disrupted intimal flaps and a subtle ulcerated palque (arrow) that was not clearly seen in the corresponding IVUS image. C: an obvious intimal rupture (arrow) with large cavitary change (*) was also clearly identified by OCT compard with corresponding IVUS image.
References
1. Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006. 151:1260–1264.
2. Doyle B, Rihal CS, O'Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007. 116:2391–2398.
3. Fineschi M, Carrera A, Gori T. Atheromatous degeneration of the neointima in a bare metal stent: intravascular ultrasound evidence. J Cardiovasc Med (Hagerstown). 2009. 10:572–573.
4. Baek EK, Park SH, Kwon KH, Shim EJ, Jo JY. A case of in-stent plaque rupture presenting as an acute myocardial infarction. Korean Circ J. 2008. 38:432–435.
5. Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol. 2006. 97:1172–1175.
6. Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002. 39:604–609.
7. Komatsu R, Ueda M, Naruko T, Kojima A, Becker AE. Neointimal tissue response at sites of coronary stenting in humans: macroscopic, histological, and immunohistochemical analyses. Circulation. 1998. 98:224–233.
8. Sangiorgi G, Taylor AJ, Farb A, et al. Histopathology of postpercutaneous transluminal coronary angioplasty remodeling in human coronary arteries. Am Heart J. 1999. 138:681–687.
9. Chung IM, Gold HK, Schwartz SM, Ikari Y, Reidy MA, Wight TN. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol. 2002. 40:2072–2081.
10. Lee CW, Kang SJ, Park DW, et al. Intravascular ultrasound findings in patients with very late stent thrombosis after either drug-eluting or bare-metal stent implantation. J Am Coll Cardiol. 2010. 55:1936–1942.
11. Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011. 57:1314–1322.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download