Abstract
The Amyloid β peptide (Aβ) is a main component of senile plaques in Alzheimer's disease. Currently, NADPH oxidase (NOX) and mitochondria are considered as primary sources of ROS induced by Aβ. However, the contribution of NOX and mitochondria to Aβ-induced ROS generation has not been well defined. To delineate the relative involvement of NOX and mitochondria in Aβ-induced ROS generation and neuronal death in mouse cortical cultures, we examined the effect of NOX inhibitors, apocynin and AEBSF, and the mitochondria-targeted antioxidants (MTAs), mitotempol and mitoquinone, on Aβ-induced ROS generation and neuronal deaths. Cell death was assessed by measuring lactate dehydrogenase efflux in bathing media at 24 and 48 hrs after exposure to Aβ1-42. Aβ1-42 induced dose- and time-dependent neuronal deaths in cortical cultures. Treatment with 20 µM Aβ1-42 markedly and continuously increased not only the DHE fluorescence (intracellular ROS signal), but also the DHR123 fluorescence (mitochondrial ROS signal) up to 8 hrs. Treatment with apocynin or AEBSF selectively suppressed the increase in DHE fluorescence, while treatment with mitotempol selectively suppressed the increase in DHR123 fluorescence. Each treatment with apocynin, AEBSF, mitotempol or mitoquinone significantly attenuated the Aβ1-42-induced neuronal deaths. However, any combined treatment with apocynin/AEBSF and mitotempol/mitoquinone failed to show additive effects. These findings indicate that 20 µM Aβ1-42 induces oxidative neuronal death via inducing mitochondrial ROS as well as NOX activation in mixed cortical cultures, but combined suppression of intracellular and mitochondrial ROS generation fail to show any additive neuroprotective effects against Aβ neurotoxicity.
Alzheimer disease (AD), the most common age-related dementia, is characterized by progressive cognitive decline and changes in personality. AD is pathologically characterized by an increase in the number of extracellular amyloid beta-peptide (Aβ)-rich senile plaques, an increase in intracellular neurofibrillary tangles composed of aggregated hyper-phosphorylated Tau, a microtubule stabilizing protein, and losses of synapses and cortical neurons. Evidence has been accumulated that oxidative stress is a major pathogenic mechanism of AD and Aβ, a main component of senile plaques, is known to be the most important causing factor of oxidative stress.123
Recently, NADPH oxidases (NOX) emerged as a novel, promising class of pharmacological targets for the treatment of neurodegeneration due to their role in oxidant generation.45 There are many reports that Aβ produced oxidative neuronal deaths by inducing NOX activation in microglia,6 astrocytes7 or neurons.8 On the other hand, it has been well-established that Aβ can interact with mitochondria and cause mitochondrial dysfunction. An immediate consequence of mitochondrial dysfunction is the increase of reactive oxygen species (ROS) production that promotesoxidative damage to DNA, RNA, proteins, and lipids.39 Therefore, it is reasonable to deliver antioxidant molecules to mitochondria to prevent the Aβ-induced oxidative damage. Recently, some mitochondria-targeted antioxidants have been developed and tested on various oxidative-stress induced disease models.1011
To delineate the relative involvement of NOX and mitochondria in Aβ-induced ROS generation and neuronal death in mouse cortical cultures, we examined the effect of NOX inhibitors, apocynin12 and 4-(2-Aminoethyl)benzenesulfonylfluoride (AEBSF),13 as well as mitochondria-targeted antioxidants (MTAs), mitotempol14 and mitoquinone,15 on the Aβ-induced ROS generation and neuronal deaths. In addition to this, we also tested if the combined treatments with NOX inhibitors and MTAs show any synergistic effect on the neuronal deaths.
Mixed cortical cell cultures, including both neurons and glia, were prepared with minor modification of methods previously described by Choi et al.16 Pregnant ICR mice (Damool, Korea) at 15–17 days of gestation were euthanized by cervical dislocation under halothane anesthesia. Fetal mice were rapidly removed and decapitated, mouse brains were excised and then rinsed in cold Ca2+/Mg2+-free Hanks' balanced salt solution supplemented with 5 mg/ml glucose, 7 mg/ml sucrose, and 0.35 mg/ml sodium bicarbonate (DM). Using fine-tipped forceps and a microsurgical knife, the meninges were carefully removed from the brain tissue under a stereomicroscope. The cerebral cortex was dissected free and minced into 1–2 mm3 sized pieces with a sterile scalpel. The cortex pieces were incubated in DM adding 0.25% trypsin at 37℃ for 15 minutes and centrifuged at 1,000×g for 5 minutes. After removal of supernatant, the tissue pellet was suspended in 1–2 mL plating medium with Eagle's minimal essential medium (MEM) containing 2 mM glutamine, 5% fetal bovine serum (FBS), and 5% horse serum (HS). Cells were separated in 8 or 10 trituration passages using a flame-narrowed pipette. Dissociated cortical cells were plated onto the previously established glial layer in 24-well multi-well plates at a density of 3 hemispheres/plate (approximately 2.5×105 cells per well). The plates were placed in an incubator (Forma, USA) at 37℃, 5% CO2, with humidified air. Cytosine arabinoside was added to produce a final concentration of 10 µM at 5 days in vitro (DIV) and maintained for 2 days to halt non-neuronal cell division. The culture medium was changed twice a week after 7 DIV. Cultures were used at 13 or 14 DIV for the experiments.
Cortical glial cultures were prepared from postnatal ICR mice (Damool, Korea) aged 1–2 days. Dissection and dissociation were as described above for mixed cortical cell cultures, and cells were plated in 24-well multi-well plates at a density of 0.5 hemisphere/plate. The plating medium was supplemented with Eagle's MEM containing 2 mM glutamine, 10% FBS, 10% HS, and 10 ng/mL of epidermal growth factor. The plates were maintained in the same incubator. The culture medium was changed once a week after 14 DIV. Glial cultures were used for the plating of mixed cortical cell cultures between 18 and 24 DIV.
The procedures involving experimental animals complied with the regulations for the care and use of laboratory animals of the animal ethical committee of Chonnam National University.
After washing with MEM (with Earle's salts), cultures were exposed to Aβ1-42, and any drugs for 24 or 48 hrs. Each row of the 24-well plates had 4 wells that received the same treatment. The four wells in the first row were treated with sham wash, NMDA (500 µM) was used to kill all of neurons in the second row, and the third to the sixth rows were treated with drugs 4 to 8 µl in each well with culture media.
The Aβ1-42 peptide was dissolved in 1,1,1,3,3,3-hexa-fluro-2-propanol (HFIP, sigma) to 1 mM, incubated at 23℃ for 60 min plus 10 min on ice and the solution was allowed to evaporate. The dried films were stored at −80℃. For oligomer preparation, films were resuspended to 5 mM Aβ in DMSO, diluted to 100 µM in cold sterile phosphate-buffered saline (PBS) and incubated at 4℃ for 24 hrs. The preparation was centrifuged at 14000 g for 10 min at 4℃ to remove insoluble aggregates and the supernatant containing soluble Aβ oligomers was transferred to clean tubes and stored at 4℃. Oligomer solutions were used within 24 hrs of preparation.
Cell death was morphologically assessed under a phase-contrast microscope, and a quantitative assessment of cell death was then assessed by measuring the activity of lactate dehydrogenase (LDH) in bathing media at 24 or 48 hrs after treatment. The sample medium (25 µl) was taken and diluted by mixing with 125 µl of reaction buffer and mixing again with 100 µl of 0.3 mg/ml NADPH and 30 µl of 22.7 mM pyruvate solutions. Absorbance changes at 340 nm were monitored for 3 min immediately after mixing with the pyruvate solution by a microplate reader (Molecular Devices, USA). The standard enzyme was purchased from Sigma-Aldrich (USA). Each LDH value, after subtracting the mean background value in control non-treated cultures (=0), was scaled to the mean value in positive control cultures treated with 500 µM NMDA for 24 h (=100), which induced near complete neuronal death in the absence of glial damage. The data is shown as percentages (mean±SEM) of the activity values from the NMDA-treated cell group (full kill).
For morphological evaluation of nuclei, SYTOX Green (Invitrogen, USA) staining was used. After fixing cells with 4% paraformaldehyde for 40 min at room temperature, the cells were permeabilized with 0.5% triton X-100 for 10 min, stained with 1 µM SYTOX Green for 15 min, and mounted with a SlowFade Antifade kit (Molecular Probes, USA). Changes in nuclear shapes and patterns were observed, and photographs were taken under a fluorescent microscope (Ex/Em=504/523nm).
Intracellular and mitochondrial reactive oxygen species (ROS) were respectively examined using dihydroethidium (DHE, Cayman, USA)17 and dihydrorhodamine 123 (DHR123, Cayman, USA).18 Cell were loaded with 5 µM DHE and 10 µM DHR123 for 30 min and then treated with amyloid beta alone or in combination with NOX inhibitors and/or MTAs. After treatments, ROS generation was monitored in a SpectraMax Gemini XPS microplate reader (Molecular devices, USA) with excitation at 530 nm and emission at 590 nm for DHE or excitation at 500 nm and emission at 536 nm for DHR123. Each fluorescence value was obtained by subtracting the mean background value of the sham-treated control cultures. In some cases, cultures were viewed under fluorescence microscopy and photographed.
Media for cell culture were purchased from Gibco BRL (Rockville, MD, USA). Fetal bovine serum and horse serum were from Hyclone (Logan, UT, USA) which inactivated at 55℃ for 30 min for use. HEPES (acid), glucose, NaHCO3, NaCl, KCl, MgCl2, CaCl2, NaOH, phenol red, trypsin, cytosine arabinoside, epidermal growth factor, sucrose, ascorbate, NMDA, and trolox were purchased from Sigma-Aldrich. Apocynin, AEBSF, and N-benzyloxycarbonyl-Val-Ala-Aspfluoromethylketone (z-VAD-FMK) were obtained from Calbiochem Corporation (San Diego, CA, USA). BDNF (Upstate Biotechnology, USA), Aβ1-42 (Bachem Americas, Inc. Torrance, CA, USA), mitotempol (Abcam, Cambridge, UK), and mitoquinone (Biovision, Milpitas, CA, USA) were used.
Results are expressed as mean±SEM values, and the differences between effects were statistically evaluated using one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls post hoc tests using Instat software (Graphpad Software Inc., USA). Statistical significance was accepted at p values <0.05.
Mixed cortical cultures were exposed to Aβ1-42 for 24 and 48 hrs at selected concentrations. The neuronal death was quantitatively assessed by measuring LDH activity in media. The neuronal death increased from 15 and 28% to 30 and 51% and 45 and 76% at 24 hrs and 48 hrs while increasing the concentrations from 5 µM to 10, 20 µM, respectively (Fig. 1A). The 20 µM Aβ1-42 induced chromatin condensation and nuclear fragmentation, hallmarks of apoptosis, shown by Sytox-green nuclear staining (Fig. 1B, upper panel). In addition to the morphological apoptotic features, treatments with anti-apoptotic drugs, such as z-VAd-fmk (ZVAD, 100 µM, a pan-caspase inhibitor) and BDNF (100 nM, brain-derived neurotrophic factor), also significantly attenuated the neuronal death induced by 20 µM Aβ1-42 (Fig. 1B, lower panel). To examine the involvement of oxidative stress and NOX activation in the neuronal death, we investigated the effect of antioxidants (Fig.1C, 100 µM trolox and 100 µM ascorbic acid) and NOX inhibitors (Fig. 1D, 500 µM apocynin or 50 µM AEBSF) on the 20 µM Aβ1-42-indued neuronal death. All four drugs showed significant inhibition of neuronal death (Fig. 1C, D).
Next, we tried to monitor generation of intracellular and mitochondrial ROS in the cells treated with Aβ1-42, directly, employing DHE, a fluorescent indicator for intracellular ROS, and DHR123, a fluorescent indicator for mitochondrial ROS, respectively. We monitored the changes in fluorescent signals for 8 hrs after exposure to 20 µM Aβ1-42. As shown in Fig. 2, treatment with 20 µM Aβ1-42 markedly and continuously increased not only the DHE fluorescent signals (Fig. 2A, B) but also the DHR123 fluorescent signals (Fig. 2C, D) for up to 8 hrs. This data implicated that 20 µM Aβ1-42 induces the generation of mitochondrial ROS as well as intracellular ROS. To evaluate the relative selectivity of NOX inhibitors and MATs for inhibiting DHE and DHR123 fluorescence, we examined the effect of apocynin, AEBSF and/or mitotempol on either DHE or DHR123 fluorescent signals. As shown in Fig. 2A and B, the treatments with 500 µM apocynin or 50 µM AEBSF markedly suppressed the Aβ-induced DHE fluorescent signals by 66 or 71 % respectively, while the treatment with 300 nM mitotempol partially suppressed the signals by 26 %. Furthermore, the combined treatments with mitotempol and apocynin/AEBSF did not show any additive effect. Conversely, the treatments with 500 µM apocynin or 50 µM AEBSF partially suppressed the Aβ-induced DHR123 fluorescent signals by 33 or 26 % respectively, while the treatment with 300 nM mitotempol markedly suppressed the DHR123 signals by 68 %. Additionally, the combined treatments with mitotempol and apocynin/AEBSF did not show a significant, additive effect (Fig. 2C, D). This data suggested that apocynin/AEBSF and mitotempol have a selective inhibitory effect on intracellular and mitochondrial ROS generation, respectively. Since Nox inhibitors and MTAs showed suppressive effects on Aβ-induced production of intracellular and mitochondrial ROS, we further examined if the combined treatments with NOX inhibitors and MTAs showed any synergistic effect on neuronal death. As shown in Fig. 3, each treatment with apocynin (500 µM), AEBSF (50 µM), mitotempol (300 nM) or mitoquinone (300 nM) significantly attenuated the 20 µM Aβ1-42-induced neuronal deaths. However, any combined treatment with apocynin/AEBSF and mitotempol/mitoquinone failed to show additive effects. When cortical neurons were exposed to 20 µM Aβ1-42 for 24 hrs or 48 hrs, the amounts of LDH released in the culture media were 44±1.4% (n=12) and 76±2.5% (n=12), respectively. Pretreatment with apocynin, mitotempol or a combination of both drugs significantly reduced the LDH release to 15±1.6%, 34±1.9% or 20±0.7 for 24 hrs (n=12), and 33±1.8%, 45±2.1% or 35±2.2% for 48 hrs (n=12), respectively (p<0.05) (Fig. 3A). Pretreatment with AEBSF, mitotempol or a combination of both drugs significantly reduced the LDH release from 48±2.0% (n=8) for 24 hrs and 68±2.6% for 48 hrs to 13±2.1%, 30±2.1% or 12±1.4 for 24 hrs (n=8), and 23±1.3%, 44±1.5% or 19±1.2% for 48 hrs (n=8), respectively (p<0.05) (Fig. 3B). Pretreatment with apocynin, mitoquinone or combination of both drugs significantly reduced the LDH release from 47±1.9% (n=8) for 24 hrs and 66±2.4% for 48 hrs to 15±0.7%, 30±1.9% or 14±0.9 for 24 hrs (n=8), and 32±2.2%, 45±1.6% or 31±2.7% for 48 hrs (n=8), respectively (p<0.05) (Fig. 3C). Pretreatment with AEBSF, mitoquinone or a combination of both drugs significantly reduced the LDH release from 46±1.9% (n=8) for 24 hrs and 68±2.6% for 48 hrs to 13±2.3%, 32±2.1% or 11±1.6 for 24 hrs (n=8), and 21±2.3%, 42±1.9% or 18±1.5% for 48 hrs (n=8), respectively (p<0.05) (Fig. 3D).
Aβ peptides, a hallmark of senile plaque of AD, are composed of 40 (Aβ1-40) or 42 (Aβ1-42) amino acid residues. Aβ1-40 represents the most abundant form of Aβ in the brain, while Aβ1-42 shows a significant increase with certain forms of AD. Aβ1-42 has two extra hydrophobic amino acids compared to Aβ1-40, which promotes greater fibrillar formation in Aβ1-42 and is known to be more toxic.19 On the other hand, Aβ25-35, a β-amyloid peptide fragment, has been widely used in both in vitro and in vivo models for AD research, because of equivalent neurotoxic action and easier availability.8 Recently, many reports have suggested that soluble Aβ oligomers are more toxic than fibrillar ones are and that the soluble oligomers, and not the plaques, correlate well with cognitive decline.320 In this study, we used Aβ1-42 oligomers, and Aβ1-42 induced dosage- and exposure time-dependent neuronal death with morphologic and pharmacologic apoptotic features (Fig. 1A, B). These results are in agreement with many preceding reports that Aβ induced apoptotic neuronal deaths in concentration- and time-dependent manners.82122
Treatment with antioxidants (trolox and ascorbic acid) or NOX inhibitors (apocynin or AEBSF) significantly inhibited the 20 µM Aβ1-42-induced neuronal death (Fig. 1C, D). These findings indicate Aβ1-42 induces not only ROS generation, but also the activation of NOX. It has been well known that oxidative damage by ROS and NOX activation may be involved in Aβ-induced neuronal death. For example, some different kinds of antioxidants may prevent Aβ neurotoxicity.1623 Activation of NADPH oxidase was also observed in Alzheimer's disease brain,624 and NADPH oxidase participates in the neuronal death induced by the beta-amyloid peptide.825
In this study, treatment with 20 µM Aβ1-42 markedly and continuously increased not only the DHE fluorescence, but also DHR123 fluorescence for up to 8 hrs after the treatment. On the other hand, treatment with apocynin or AEBSF, a NOX inhibitor, selectively suppressed the increase in DHE fluorescence, while treatment with mitotempol, an MTA, selectively suppressed the increase in DHR123 fluorescence (Fig. 2A–D). This data indicates that 20 µM Aβ1-42 induces the generation of mitochondrial ROS as well as intracellular ROS, and suggests that apocynin/AEBSF and mitotempol have selective inhibitory effects on intracellular and mitochondrial ROS generation, respectively. There is some evidence that not only NOX activation, but mitochondrial ROS are involved in proinflammatory microglial activation.26 Recently Wang et al.27 reported the involvement of NOX- and mitochondria-derived ROS in the process of Aβ1-40-induced NLRP3 inflammasome activation in LPS-primed ARPE-19 cells in the age-related macular degeneration model. From these findings, we tried to investigate the effects of the combined treatment with NOX inhibitors, apocynin or AEBSF and MTAs, mitotempol or mitoquinone, on Aβ1-42-induced neuronal death. So far, we could not find any papers which examine effect of combined treatment with NOX inhibitors and MTAs on any markers related with oxidative stress or cell death.
Our hypothesis for the combined treatment was that if NOX-induced intracellular ROS generation and the mitochondrial ROS generation were independently induced by the Aβ1-42 treatment, they would induce neuronal death independently. Unfortunately, we failed to get any additive effects with the combined treatment of NOX inhibitors and MATs (Fig. 3).
This negative result of combined treatment indicates that ROS generation from NOX activation and mitochondrial dysfunction may be sequential or inter-related, or that they may use common pathways in the cell death process. It is inappropriate to mention the interrelationship between ROS generations from NOX activation and mitochondria and the cell death process induced by ROS generation because of the limited data in the present study. Further study is required to further define these processes. On the other hand, the selectivity of the agents, DHE and DHR123, apocynin and AEBSF, mitotempol and mitoquinone, used in present study may not be good enough to generate an additive effect. Recently, new selective NOX inhibitors have been developed as drugs for neurodegenerative disorders.4 More study using these new drugs is needed.
Although we failed to show any additive protective effects of the combined treatment, this study shows that 20 µM Aβ1-42 induces oxidative neuronal death via inducing mitochondrial ROS as well as NOX activation in mixed cortical cultures.
Figures and Tables
FIG. 1
Aβ1-42-induced neuronal death in mouse cortical cultures and protective effects of antioxidants and NADPH oxidase inhibitors. (A) Aβ1-42-induced concentration- and time-dependent neuronal death. Cell death was measured by assay for lactate dehydrogenase (LDH) activity leaked out to the media and showed as percent LDH activity of NMDA-treated cell group (% LDH release). Each point and bar are the mean±SEM from 8–20 wells. (B)-up. Fluorescent photomicrographs from typical representative fields (200×field) of cells were taken after a 18-hour exposure to sham wash (sham) or 20 µM Aβ1-42. (Aβ). Arrows indicate the fragmented and condensed chromatin stained with Sytox green. (B)-low. Effect of treatment with 100 µM z-VAD-fmk (+ZVAD) and 100 nM BDNF on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. Each column and bar are the mean±SEM from 8–12 wells. (C) Effect of treatment with trolox (100 µM) and ascorbic acid (100 µM) on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. Each column and bar are the mean±SEM from 8–12 wells. (D) Effect of treatment with apocynin (500 µM), AEBSF (50 µM) on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. Each column and bar are the mean±SEM from 8–12 wells. *Significantly different from corresponding Aβ-treated control group (p<0.05).
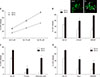
FIG. 2
Time-course of intracellular (DHE fluorescence, A, B) and mitochondrial (DHR fluorescence, C, D) reactive oxygen species (ROS) generation by 20 µM Aβ1-42 and the effects of treatment with apocynin (500 µM), AEBSF (50 µM), and mitotempol (300 nM) on the Aβ1-42-induced ROS generation in mouse mixed cortical cultures. Intracellular and mitochondrial ROS were respectively examined using dihydroethidium (DHE) and dihydrorhodamine 123 (DHR123). Cell were loaded with 5 µM DHE and 10 µM DHR123 for 30 min and then treated with Aβ1-42 alone or in combination with apocynin/AEBSF and mitotempol. After treatments, ROS generation was monitored in a spectrophotometer with excitation at 530 nm and emission at 590 nm for DHE or excitation at 500 nm and emission at 536 nm for DHR123. Each fluorescence value was obtained by subtracting the mean background value of sham-treated control cultures. Each point and bar is the mean±SEM from 8–12 wells. *Significantly different from corresponding Aβ-treated control group (p<0.05). **Significantly different from corresponding Aβ-treated control group (p<0.01).

FIG. 3
Effects of single or combined treatment with NADPH oxidase inhibitors and mitochondria-targeted antioxidants on the 20 µM Aβ1-42-induced neuronal death in mouse cortical cultures. (A) Effects of single or combined treatment with 500 µM apocynin or 300 nM mitotempol on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. (B) Effects of single or combined treatment with 50 µM AEBSF or 300 nM mitotempol on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. (C) Effects of single or combined treatment with 500 µM apocynin or 300 nM mitoquinone on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. (D) Effects of single or combined treatment with 50 µM AEBSF or 300 nM mitoquinone on the 20 µM Aβ1-42-induced neuronal death at the end of 24 hrs and 48 hrs exposure. Cell death was measured the same as in Fig. 1. Each column and bar are the mean±SEM from 8–12 wells. Any combined treatments with NADPH oxidase (NOX) inhibitors and mitochondria-targeted antioxidants on the 20 µM Aβ1-42-induced neuronal death failed to show statistically significant from any corresponding treatment with NOX inhibitor. *Significantly different from corresponding Aβ-treated control group (p<0.05).

References
2. Butterfield DA. beta-amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer's disease. Chem Res Toxicol. 1997; 10:495–506.

3. Butterfield DA, Swomley AM, Sultana R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013; 19:823–835.

4. Sorce S, Stocker R, Seredenina T, Holmdahl R, Aguzzi A, Chio A, et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: what is the evidence? Free Radic Biol Med. 2017; 112:387–396.

5. Cifuentes-Pagano ME, Meijles DN, Pagano PJ. Nox inhibitors & therapies: rational design of peptidic and small molecule inhibitors. Curr Pharm Des. 2015; 21:6023–6035.

6. Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, et al. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010; 12:1371–1382.

7. Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005; 25:9176–9184.

8. Chay KO, Nam Koong KY, Hwang S, Kim JK, Bae CS. NADPH oxidase mediates β-amyloid peptide-induced neuronal death in mouse cortical cultures. Chonnam Med J. 2017; 53:196–202.

9. Picone P, Nuzzo D, Caruana L, Scafidi V, Di Carlo M. Mitochondrial dysfunction: different routes to Alzheimer's disease therapy. Oxid Med Cell Longev. 2014; 2014:780179.

10. Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med. 2011; 11:106–114.
12. Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006; 1090:182–189.

13. Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997; 272:13292–13301.

14. Trnka J, Blaikie FH, Logan A, Smith RA, Murphy MP. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic Res. 2009; 43:4–12.

15. Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007; 9:1825–1836.

16. Choi SM, Kim BC, Cho YH, Choi KH, Chang J, Park MS, et al. Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med J. 2014; 50:45–51.

17. Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012; 52:1–6.

18. Nagahara Y, Shiina I, Nakata K, Sasaki A, Miyamoto T, Ikekita M. Induction of mitochondria-involved apoptosis in estrogen receptor-negative cells by a novel tamoxifen derivative, ridaifen-B. Cancer Sci. 2008; 99:608–614.

19. Naslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, et al. Relative abundance of Alzheimer a beta amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci U S A. 1994; 91:8378–8382.

20. Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB Life. 2007; 59:332–345.

21. Han XJ, Hu YY, Yang ZJ, Jiang LP, Shi SL, Li YR, et al. Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol Med Rep. 2017; 16:4521–4528.

22. Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem. 2002; 277:15666–15670.

23. Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur J Neurosci. 2000; 12:1882–1890.

24. Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, et al. Activation of NADPH oxidase in Alzheimer's disease brains. Biochem Biophys Res Commun. 2000; 273:5–9.

25. Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos Trans R Soc Lond B Biol Sci. 2005; 360:2309–2314.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download