Abstract
Positron emission tomography (PET) /computed tomography (CT) has been established as a standard imaging modality in the evaluation of malignancy. Although PET/CT has played a major role in the management of oncology patients, its clinical use has also increased for various disorders other than malignancy. Growing evidence shows that PET/CT images have many advantages in aortic disease as well. This review article addresses the potential role of PET/CT in diseases involving the aorta, emphasizing its usefulness with regard to acute thoracic aortic syndromes, aortic aneurysm, atherosclerotic lesions, aortitis and aortic tumors.
Aortic diseases are disorders of the aorta that include acute aortic syndromes, aortic aneurysms, atherosclerosis, inflammatory diseases, and aortic tumors. They can be detected early based on acute symptoms or diagnosed much later. Aortic diseases, particularly acute aortic syndromes, require the swift identification or exclusion of diseases, which affect patient prognosis.
Several imaging modalities have been used to determine the presence and progression of aortic diseases. Chest x-rays can detect abnormalities in aortic contour or size,1 but chest x-rays are inadequate for excluding the presence of aortic dissection and screening for chest trauma.23 Considering the advantages of universal availability, rapid acquisition, and high resolution of computed tomography (CT), CT has thus far played a central role in the diagnosis, risk stratification, and management of aortic disease, particularly in emergency settings.4 In contrast to CT, which lacks the ability to show crucial differences in physiology, nuclear medicine imaging has the advantages of providing molecular and functional information. In recent years, as technology has developed, combined Positron Emission Tomography/Computed Tomography (PET/CT) has been adopted for offering both the high resolution of CT and the ability of PET to show the molecular aspect; thus, integrated PET/CT images can simultaneously reveal anatomical, molecular, and functional information. Consequently, PET/CT improves the diagnostic accuracy and localization of many lesions.5 Since South Korea began reimbursement via a public insurance system in June 2006,6 the number of PET studies has greatly increased. Moreover, as the market for PET/CT devices increases, the clinical application of PET/CT is expanding beyond malignant diseases. South Korea has adopted broad 18F-fluorodeoxyglucose (FDG) PET/CT indications, including assessment of malignancy, ischemic heart disease, partial-onset seizure, and other diseases that may benefit from its use. Europe and the United States have also accepted a relatively broad spectrum of indications. Therefore, there is an increasing trend in the use of FDG PET/CT for diseases other than malignancy.
This article provides an overview of recent PET/CT studies in the field of aortic disease. Seeing as PET/CT imaging has been applied in a wide range of clinical settings, its use has increased in the evaluation of diseases involving the aorta and arteries. Although the usefulness of PET/CT has not been established in the field of aortic disease, we summarize previous studies on PET/CT, and address its potential role in diseases involving the aorta and arteries, including the acute thoracic aortic syndromes, aortic aneurysm, atherosclerotic lesions, aortitis and aortic tumors.
Acute thoracic aortic syndromes encompass a spectrum of emergency conditions, including aortic dissection, intramural hematomas and penetrating atherosclerotic ulcers. They are life-threatening conditions that require prompt diagnosis. CT is the imaging modality of choice for suspected dissection and in general in cases of acute aortic syndromes,78 and emergent operations are performed according to CT images. In contrast to CT, the major drawback of FDG PET/CT is its prolonged scan time. The acquisition of PET/CT starts 60 minutes after FDG administration, not immediately, however, the interval between FDG administration and the start of acquisition can be shortened to some extent.9 Nevertheless, in an emergency, it is unethical to delay surgery for FDG PET/CT diagnosis, and it is thus prohibited. Obviously, FDG PET/CT is not included in recent guidelines regarding acute aortic syndromes.78 Therefore, most previous studies have retrospectively reviewed images in cases of aortic dissection or in cases where an emergent operation could not be performed because of the greater severity of the patient's accompanying disease. Considering the disadvantages of a prolonged scan time, which obviates its applicability in acute settings, FDG PET/CT studies are relatively few in number. Very few studies have been conducted on cases of Stanford type A aortic dissection that need emergent surgical repair.10
FDG PET/CT may help to discriminate acute from chronic aortic dissection.11 Acute dissection of the aortic wall leads to elevated metabolic activity at the site of fresh laceration of the aortic wall, and acute vascular injury may induce repair mechanisms leading to accumulation of glycolytic active cells, such as macrophages and activated myofibrinocytes in the vessel wall, thus enhancing FDG uptake. Reeps et al.11 suggested that FDG PET/CT can be helpful in the diagnosis of asymptomatic acute dissection, which results in severe complications. The standardized uptake value (SUV) ratio and SUVmax can overcome the low specificity of electrocardiograms and laboratory parameters such as fibrinogen and D-dimers, to exclude acute dissection. Furthermore, FDG PET/CT imaging may be helpful in identifying patients with acute aortic syndromes with an increased risk of disease progression. Kuehl et al.12 reported vessel wall inflammation in one-third of examined patients with acute aortic syndromes, and this patient group seemed to have a high risk for disease progression. A case study that reviewed data from serial cancer-screening PET/CT obtained 5 years prior to acute aortic events revealed a trend of gradually increasing aortic FDG activity when the dissection was imminent.13 Kato et al.10 showed that SUVmax and SUVmean were significantly greater for the unfavorable outcome group than for the favorable outcome group. A mean SUV greater than 3.029 demonstrated significantly greater predictive power for acute aortic dissection, and greater FDG uptake in the dissected aortic wall was significantly associated with an increased risk for rupture and progression of aortic dissection. Unlike CT scans, follow-ups with serial FDG PET/CT may be able to detect pathologic changes in the dissected aorta and to clarify the pathologic mechanism of the disappearance or regression of acute aortic dissection.1014 However, the revised 2014 ACR Appropriateness Criteria15 rated FDG PET/CT scans with a score of 3 for acute chest pain - suspected aortic dissection, which typically indicates an inappropriate modality.15 Nevertheless, FDG PET/CT may play a role in prognosticating the outcome in individuals diagnosed with aortic dissection and further investigation is warranted.
Intramural hematomas and penetrating atherosclerotic ulcers are components of acute aortic syndromes. A few PET/CT case reports have been published on intramural hematoma, acute intramural hematoma of the aorta with positive FDG uptake,16 acute intramural hematoma of the ascending aorta,17 intramural hematoma at a pulmonary artery bifurcation site and the ascending aorta,18 and intramural hematomas with penetrating ulcer and saccular pseudoaneurysm in the abdominal aorta.19 These cases exhibited significant FDG uptake with an SUV of 4.4 to 10 in the lesions. In accordance with intramural hematomas, penetrating aortic ulcers exhibited increased glucose metabolism within the penetrating ulcer.20 Patients with penetrating aortic ulcers who exhibited greater FDG uptake in the aortic wall were more likely to develop adverse aortic events, and a target-to-background ratio of 1.5 had an acceptable predictive value for differentiating high-risk from low-risk patients.21 Gorla et al.22 demonstrated that pathological glucose uptake in aortic wall lesions determined using FDG PET/CT was associated with high C-reactive protein (CRP) levels, increased mortality, and more major adverse events at 3-year follow-ups in acute aortic syndromes including penetrating aortic ulcers.
In summary, FDG PET/CT for acute aortic syndrome plays a potential role in diagnosis and risk stratification, discrimination of acute from chronic dissection, prediction of development of aortic dissection, and prediction of unfavorable outcomes including rupture and progression.
Aortic aneurysm is an enlargement of the aorta of up to 1.5 times the normal size. Aortic aneurysms cause weakness in the wall of the aorta and increase the risk of aortic rupture. Studies conducted before 2010 reported many cases with FDG uptake in the aortic aneurysm wall;2324 hence, FDG PET/CT was introduced as a new diagnostic technique to study aortic aneurysm disease in vivo.25 Sakalihasan et al.23 reported several cases that required emergent or urgent aneurysmectomy for ruptured, leaking, rapidly expanding, or painful aneurysms with FDG uptake. In a surveillance study of small aortic aneurysms,26 the majority of non-specific aortic aneurysm patients exhibited increased FDG uptake with a SUVmax of over 2.5. Histologic study showed that increased FDG uptake in the aortic aneurysm wall was correlated with higher densities of inflammatory infiltrates and macrophage, T-cell infiltrations, and lower collagen fiber content and vascular smooth muscle cell density.25 Thus, FDG PET/CT may contribute to improved prediction of aneurysmal rupture risk. Another study also demonstrated that FDG uptake in the aortic aneurysmal wall displayed alterations potentially related to medial degeneration and considerable degradation of the fibrillary structures of the adventitia.27 They showed that the FDG uptake is related to the massive infiltration of activated lymphocytes and macrophages in the aneurysmal wall, inducing profound remodeling. In addition, FDG activity was commonly focused in the shoulder region of the aneurysmal wall, which is possibly related to a destructive macrophage phenotype; accordingly, it is considered to provide information different from that obtained from an MRI.28 Furthermore, high stress to aortic aneurysm wall may also be linked with high levels of FDG uptake.29
However, studies conducted after 2010 have also addressed aortic aneurysm in asymptomatic patients, and they have consistently shown that FDG uptake in aortic aneurysm is not a common finding. Tegler et al.30 reported no significantly increased FDG uptake in asymptomatic aneurysmal patients compared with healthy controls, although histologic examination of the aneurysm walls showed high inflammatory cell infiltration by T lymphocytes, B lymphocytes, and macrophages. Palombo et al.31 assessed the prevalence of increased FDG uptake in aneurysmal walls in asymptomatic patients with aortic aneurysms, and found that increased FDG uptake was extremely rare in patients with aortic aneurysms with a diameter close to the indication for surgery. Severe loss of wall structure and a low number of cells may explain the lack of visible FDG uptake in asymptomatic patients with aortic aneurysms, the diameter of which is close to the indication for surgery.32 Another longitudinal PET/CT study, in line with recent studies, showed that aortic aneurysms with lower FDG uptake may be more likely to expand.33 Barwick et al.34 also demonstrated no correlation between aneurysmal size and metabolic activity in a relatively large series with an unselected and well-matched control group. Previous histologically validated studies have shown that increased FDG uptake on PET/CT predicts the degree of wall inflammation,232526 but currently increasing studies are reporting that FDG uptake varies widely in the process of aneurysmal expansion and the results for FDG uptake as a predictor of clinical outcomes such as expansion or rupture are inconsistent.
Thus, because of the inconsistent results regarding FDG PET/CT, preclinical and clinical studies using various radiopharmaceuticals that can target different disease processes are currently underway to monitor the biological activity of aortic aneurysms. 68Ga-DOTATATE,35 11C-PK11195,36 18F-cross-linked iron oxide nanoparticle37 and 18F-choline383940 can target macrophages, while 18F-fluciclatide,41 18F-FPPRGD42 and 64Cu-NOTA-TRC105-Fab43 can be used for neovascularization and angiogenesis in the process of aortic aneurysms. Another radiopharmaceutical targeting microcalcification,44 18F-Sodium fluoride (NaF), has critical clinical application in aortic aneurysms. The Sodium Fluoride Imaging of Abdominal Aortic Aneurysms (SoFIA) study demonstrated both that NaF uptake identifies advanced aneurysmal disease and its association with aneurysm growth and clinical events.45 Many radiopharmaceuticals are being developed to target the various pathogenic mechanisms of aortic aneurysm progress; therefore, it is expected that PET/CT using various tracers will offer a clearer understanding of aortic aneurysms.
In summary, FDG PET/CT of aortic aneurysms has the potential to predict expansion and progress in symptomatic patients, but FDG uptake varies widely in asymptomatic patients. PET/CT using new radiopharmaceuticals related to pathogenic processes will shed new light on aortic aneurysms and the additional clinical studies will strengthen the role of PET/CT for aortic aneurysms.
Atherosclerosis is a disease of the arterial wall, whereby endothelial cell damage results in the accumulation of lipids and inflammatory cells within discrete areas of the intima, eventually encroaching upon the vessel lumen.46 FDG is the most common PET tracer in atherosclerosis PET/CT imaging. A study conducted by Rudd et al.47 revealed increased FDG uptake in symptomatic carotid plaques ipsilateral to a recent stroke, marking the first human study in which atherosclerotic plaque inflammation was imaged with FDG PET. Increasing evidence has since been obtained that there is increasing evidence that FDG PET/CT can be used as a marker of plaque vulnerability to identify patients at high risk of clinical events,484950 and as a surrogate end point in clinical trials of antiatherosclerotic therapies.515253
Vascular FDG uptake mainly indicates increased macrophage activity resulting in inflammation within atherosclerotic plaques, whereas NaF is a marker of increased vascular calcification resulting in microcalcification in the vasculature. Irkle et al.44 demonstrated that NaF binds preferentially to regions of developing microcalcification in carotid atheroma, with little or no binding to non-calcific tissue types. A recent prospective study also demonstrated the ability of NaF PET to predict progression in the CT calcium score over 1 year.54 Many studies have introduced various radiopharmaceuticals targeting pathologic processes in the imaging of the vascular cell adhesion molecule (VCAM)55 of the endothelium, choline metabolism,3856 translocator protein (TSPO),3657 somatostatin receptor5859 of macrophages, monocytes and macrophages released proteases including matrix metalloproteinase (MMP) and cathepsin,6061 hypoxia62 and neoangiogenesis.636465 Based on these preclinical studies, more active clinical studies may lead to favorable results in atherosclerotic lesions.
In summary, FDG PET/CT imaging of atherosclerosis allows for the in vivo visualization of vascular inflammation, which can help evaluate plaque vulnerability and predict clinical events. Novel PET tracers, which are designed to track active calcification, inflammation, hypoxia or neoangiogenesis, may be potential markers for plaque rupture and cardiovascular risk.
Aortitis is the pathological term for the inflammation of the aortic wall.66 The classification of aortitis broadly includes noninfectious and infectious aortitis. Noninfectious aortitis can be classified into large-, medium-, and small-sized vessel vasculitides. Infectious aortitis is induced by microorganisms such as tuberculosis, syphilis, salmonella or other bacteria. Aortitis may also result from idiopathic conditions or radiation.
PET/CT has the disadvantage of limited spatial resolution, and most PET/CT studies have focused on the visualized inflammation of the aorta and the larger arteries. Large-vessel vasculitides such as Takayasu arteritis and giant cell arteritis are the most common causes of aortitis.66 Takayasu arteritis frequently involves the aorta, and its major branches. Although FDG PET/CT is not a component of the criteria of American College of Rheumatology,67 FDG PET/CT may play a role in diagnosis, differential diagnosis, the exclusion of other causes of arteritis, and the identification of optimal target sites for biopsy.686970 FDG PET/CT allows differentiation between active and inactive Takayasu arteritis, because conventional inflammatory markers such as a high erythrocyte sedimentation rate (ESR) and CRP which reflect systemic inflammation, are nonspecific for Takayasu arteritis, and immunomodulatory agents can modify these parameters.71 Furthermore, SUVmax clearly represents therapeutic effectiveness (Fig. 1); hence, FDG PET/CT is recommended for the diagnosis of Takayasu arteritis and detection of its recurrence. An Indian study reported that FDG PET/CT showed high sensitivity for detecting active diseases during immunosuppression and may help in guiding therapy and in assessing the response to immunosuppression.72 Seeing as metabolic changes precede anatomical changes, FDG PET/CT may also be useful in cases in which they are difficult to detect using CT or MRI.73
In giant cell arteritis, FDG PET/CT has been shown to be sensitive to extracranial vasculitis, but not for intracranial vasculitis because of its poor spatial resolution.74 However, extracranial involvement is common in 30% to 74% of giant cell arteritis,75 and therefore, FDG PET/CT may be helpful in localization of the extent of large-, and medium-sized vasculitis involving the aorta, and subclavian, carotid, and iliac arteries7677 as well as small-diameter extracranial involvement such as the temporal, occipital and vertebral arteries.78 Giant cell arteritis is often associated with polymyalgia rheumatica, which is an inflammatory disease around the joints;7980 accordingly, FDG PET/CT can also be useful in the diagnosis of polymyalgia rheumatica by visualizing the affected joints.77 Moreover, although temporal artery biopsy is considered the cornerstone of the diagnosis of giant cell arteritis, false negatives can represent up to 15%–70% of cases, resulting in delayed diagnosis.8182 Negative temporal artery biopsy cannot exclude the presence of giant cell arteritis; FDG PET/CT, therefore, can be considered when giant cell arteritis is clinically suspected. This is because FDG PET/CT is able to detect early inflammation without late effects such as an arterial halo on ultrasonography and aortic wall thickening or edema on MRI.83 In addition, because giant cell arteritis with increased FDG uptake in the aorta may be more prone to thoracic aortic dilatation, FDG PET/CT can be helpful to predict individual prognoses. In chronic periaortitis, FDG PET/CT is useful to evaluate disease activity,84 and has been successfully applied after immunotherapy when residual retroperitoneal tissue persists.85 FDG PET/CT also facilitates the detection of remote disease such as that seen in multifocal fibrosclerosis, occult neoplasm, or infectious processes with which retroperitoneal fibrosis may be secondarily associated.84
In contrast to large-vessel vasculitis, vasculitis of medium and small vessels has been less thoroughly studied. However, studies have reported that FDG uptake may be possible if smaller arteries are involved,76 depending on the spatial resolution of the PET/CT camera. Several studies have reported FDG uptake in diseases involving smaller arteries such as polyarteritis nodosa,7686 Wegener's granulomatosis,87 Churg-Strauss syndrome,88 or relapsing polychondritis.899091 Furthermore, FDG PET/CT may be able to differentiate between giant cell arteritis, Takayasu arteritis, and polyarteritis nodosa.76
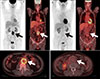
FDG PET/CT has also proven its usefulness in infectious aortitis. Several case reports have been published on infected aortic aneurysms resulting from Staphylococcus aureus92 or Salmonella enteritides.93 The site of FDG uptake within the aneurysm wall corresponds with that of indium-111 white blood cell accumulation.92 FDG PET/CT provides not only accurate information for the diagnosis of infected aortic aneurysm, but also monitors the response to antibiotic therapy.93 Murakami et al.94 demonstrated that infected aortic aneurysms, can be discriminated from infection-free cases based on an SUVmax of 4.46; therefore, FDG PET/CT is useful for the diagnosis of an infected aneurysm (Fig. 2) and determination of the effects of postoperative antibiotic therapy and completion of therapy.
In summary, FDG PET/CT is useful in noninfectious aortitis involving medium-, small-, and large-vessels. It aids in diagnosis, differential diagnosis, the exclusion of other causes of aortitis, and the identification of target sites for biopsy. Moreover, it is helpful for evaluating disease extent and activity, predicting of prognosis, monitoring the response to therapy, and evaluating therapeutic effectiveness.
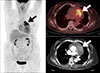
Primary malignant tumors of the aorta are extremely rare,95 and exhibit heterogeneous histopathology. Sarcomas of the intima are the most common, followed by leiosarcomas and fibrosarcomas of the media or adventitia of the aortic wall. FDG PET/CT is a useful modality for detecting malignant tumors including sarcomas of the aorta (Fig. 3).969798 In patients with peripheral or splanchnic emboli, an aortic sarcoma should be included in differential diagnosis; therefore, a whole-body PET/CT scan can be used for comprehensive staging.99 In one study of primary sarcomas of the aorta and arteries,100 FDG PET/CT showed high metabolic activity in sarcomas, local embolic spread in the pulmonary vasculature of the lungs, distant metastases to the brain, and high metabolic activity at the site of vascular filling defects, which strengthens the suspicion of malignant disease.
In summary, FDG PET/CT is useful for the evaluation of primary malignancy of the aorta, embolic spread, distant metastasis, and extension into adjacent arteries.
No research interventions were done in this review (no ethical approval or informed consent needed).
PET/CT gives useful information for the physiological, molecular, and functional aspects of aortic diseases, providing a pathophysiologic understating of acute thoracic aortic syndromes, aortic aneurysm, atherosclerotic lesions, aortitis, and aortic tumors. Patients who need diagnosis, assessment of disease extent and metabolic activity, prediction of clinical outcome, or evaluation of therapeutic effectiveness for the aortic disease could benefit from FDG PET/CT. Future studies are needed to confirm the clinical usefulness of FDG PET/CT in large patient populations and PET/CT with developing new radiopharmaceuticals should be validated for potential clinical applications in regards to the various aortic diseases.
Figures and Tables
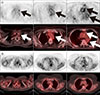
FIG. 1
Representative case of Takayasu arteritis. (A) PET and fused PET/CT images before therapy. The patient exhibited tubular 18F-fluorodeoxyglucose (FDG) uptake along the walls of the left common carotid artery, aortic arch, and ascending and descending thoracic aorta (arrows in A). (B) PET and fused PET/CT images after administration of corticosteroids. All vascular FDG uptakes were markedly improved.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea grant funded by the Korean government (NRF-2017M3A9E8023017). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002; 287:2262–2272.

2. Mirvis SE, Bidwell JK, Buddemeyer EU, Diaconis JN, Pais SO, Whitley JE, et al. Value of chest radiography in excluding traumatic aortic rupture. Radiology. 1987; 163:487–493.

3. Schwab CW, Lawson RB, Lind JF, Garland LW. Aortic injury: comparison of supine and upright portable chest films to evaluate the widened mediastinum. Ann Emerg Med. 1984; 13:896–899.

4. Salvolini L, Renda P, Fiore D, Scaglione M, Piccoli G, Giovagnoni A. Acute aortic syndromes: role of multi-detector row CT. Eur J Radiol. 2008; 65:350–358.

5. Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent). 2005; 18:321–330.

6. Kim SK, Kang K. Current status of PET in the world. In : Kim E, Lee MC, Inoue T, Wong WH, editors. Clinical PET and PET/CT. New York: Springer;2013. p. 129–135.
7. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/ SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010; 76:E43–E86.
8. Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Kardiol Pol. 2014; 72:1169–1252.

9. Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010; 37:181–200.
10. Kato K, Nishio A, Kato N, Usami H, Fujimaki T, Murohara T. Uptake of 18F-FDG in acute aortic dissection: a determinant of unfavorable outcome. J Nucl Med. 2010; 51:674–681.

11. Reeps C, Pelisek J, Bundschuh RA, Gurdan M, Zimmermann A, Ockert S, et al. Imaging of acute and chronic aortic dissection by 18F-FDG PET/CT. J Nucl Med. 2010; 51:686–691.

12. Kuehl H, Eggebrecht H, Boes T, Antoch G, Rosenbaum S, Ladd S, et al. Detection of inflammation in patients with acute aortic syndrome: comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart. 2008; 94:1472–1477.

13. Tahara N, Hirakata S, Okabe K, Tahara A, Honda A, Igata S, et al. FDG-PET/CT images during 5 years before acute aortic dissection. Eur Heart J. 2016; 37:1933.

14. Kaji S, Nishigami K, Akasaka T, Hozumi T, Takagi T, Kawamoto T, et al. Prediction of progression or regression of type A aortic intramural hematoma by computed tomography. Circulation. 1999; 100:II281–II286.

15. Jacobs JE, Latson LA Jr, Abbara S, Akers SR, Araoz PA, Cummings KW, et al. ACR appropriateness criteria® acute chest pain - suspected aortic dissection [Internet]. Rockvile (MD): Agency for Healthcare Research and Quality;c1999. cited 2018 Jul 10. Available from: https://acsearch.acr.org/docs/69402/Narrative/.
16. Ryan A, McCook B, Sholosh B, Pryma DA, Jablonowski E, Fuhrman C, et al. Acute intramural hematoma of the aorta as a cause of positive FDG PET/CT. Clin Nucl Med. 2007; 32:729–731.

17. Govaerts L, Withofs N, Durieux R, Spote V, Hustinx R. Acute intramural haematoma of the ascending aorta. Eur J Nucl Med Mol Imaging. 2012; 39:1368–1369.

18. Alves CMR, Gomes MPM Jr, Faraco RP, Sawabini T, Dias Filho PCFD, Leão Filho HM. Atypical presentation of intramural hematoma of the ascending aorta using a conservative approach. Rev Bras Cardiol Invasiva. 2014; 22:303–307.
19. Nguyen VX, Nguyen BD. PET/CT imaging of abdominal aorta with intramural hematomas, penetrating ulcer, and saccular pseudoaneurysm. Clin Nucl Med. 2014; 39:467–469.

20. Eggebrecht H, Plicht B, Kahlert P, Erbel R. Intramural hematoma and penetrating ulcers: indications to endovascular treatment. Eur J Vasc Endovasc Surg. 2009; 38:659–665.

21. Yang F, Luo J, Hou Q, Xie N, Nie Z, Huang W, et al. Predictive value of 18F-FDG PET/CT in patients with acute type B aortic intramural hematoma. J Nucl Cardiol. 2017; [Epub ahead of print]. DOI: 10.1007/s12350-017-1014-9.

22. Gorla R, Erbel R, Kuehl H, Kahlert P, Tsagakis K, Jakob H, et al. Prognostic value of (18)F-fluorodeoxyglucose PET-CT imaging in acute aortic syndromes: comparison with serological biomarkers of inflammation. Int J Cardiovasc Imaging. 2015; 31:1677–1685.

23. Sakalihasan N, Hustinx R, Limet R. Contribution of PET scanning to the evaluation of abdominal aortic aneurysm. Semin Vasc Surg. 2004; 17:144–153.

24. Takahashi M, Momose T, Kameyama M, Ohtomo K. Abnormal accumulation of [18F]fluorodeoxyglucose in the aortic wall related to inflammatory changes: three case reports. Ann Nucl Med. 2006; 20:361–364.

25. Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg. 2008; 48:417–423.

26. Kotze CW, Menezes LJ, Endozo R, Groves AM, Ell PJ, Yusuf SW. Increased metabolic activity in abdominal aortic aneurysm detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). Eur J Vasc Endovasc Surg. 2009; 38:93–99.

27. Courtois A, Nusgens BV, Hustinx R, Namur G, Gomez P, Somja J, et al. 18F-FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J Nucl Med. 2013; 54:1740–1747.

28. McBride OM, Joshi NV, Robson JM, MacGillivray TJ, Gray CD, Fletcher AM, et al. Positron emission tomography and magnetic resonance imaging of cellular inflammation in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2016; 51:518–526.

29. Xu XY, Borghi A, Nchimi A, Leung J, Gomez P, Cheng Z, et al. High levels of 18F-FDG uptake in aortic aneurysm wall are associated with high wall stress. Eur J Vasc Endovasc Surg. 2010; 39:295–301.

30. Tegler G, Ericson K, Sorensen J, Bjorck M, Wanhainen A. Inflammation in the walls of asymptomatic abdominal aortic aneurysms is not associated with increased metabolic activity detectable by 18-fluorodeoxglucose positron-emission tomography. J Vasc Surg. 2012; 56:802–807.

31. Palombo D, Morbelli S, Spinella G, Pane B, Marini C, Rousas N, et al. A positron emission tomography/computed tomography (PET/CT) evaluation of asymptomatic abdominal aortic aneurysms: another point of view. Ann Vasc Surg. 2012; 26:491–499.

32. Marini C, Morbelli S, Armonino R, Spinella G, Riondato M, Massollo M, et al. Direct relationship between cell density and FDG uptake in asymptomatic aortic aneurysm close to surgical threshold: an in vivo and in vitro study. Eur J Nucl Med Mol Imaging. 2012; 39:91–101.

33. Kotze CW, Groves AM, Menezes LJ, Harvey R, Endozo R, Kayani IA, et al. What is the relationship between 18F-FDG aortic aneurysm uptake on PET/CT and future growth rate? Eur J Nucl Med Mol Imaging. 2011; 38:1493–1499.

34. Barwick TD, Lyons OT, Mikhaeel NG, Waltham M, O'Doherty MJ. 18F-FDG PET-CT uptake is a feature of both normal diameter and aneurysmal aortic wall and is not related to aneurysm size. Eur J Nucl Med Mol Imaging. 2014; 41:2310–2318.

35. Rinne P, Hellberg S, Kiugel M, Virta J, Li XG, Kakela M, et al. Comparison of somatostatin receptor 2-targeting PET tracers in the detection of mouse atherosclerotic plaques. Mol Imaging Biol. 2016; 18:99–108.

36. Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/ computed tomography. Eur Heart J. 2012; 33:1902–1910.

37. Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011; 31:750–757.

38. Bucerius J, Schmaljohann J, Bohm I, Palmedo H, Guhlke S, Tiemann K, et al. Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans–first results. Eur J Nucl Med Mol Imaging. 2008; 35:815–820.

39. Matter CM, Wyss MT, Meier P, Spath N, von Lukowicz T, Lohmann C, et al. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscler Thromb Vasc Biol. 2006; 26:584–589.
40. Sarda-Mantel L, Alsac JM, Boisgard R, Hervatin F, Montravers F, Tavitian B, et al. Comparison of 18F-fluoro-deoxy-glucose, 18F-fluoro-methyl-choline, and 18F-DPA714 for positron-emission tomography imaging of leukocyte accumulation in the aortic wall of experimental abdominal aneurysms. J Vasc Surg. 2012; 56:765–773.

41. Tegler G, Estrada S, Hall H, Wanhainen A, Bjorck M, Sorensen J, et al. Autoradiography screening of potential positron emission tomography tracers for asymptomatic abdominal aortic aneurysms. Ups J Med Sci. 2014; 119:229–235.

42. Kitagawa T, Kosuge H, Chang E, James ML, Yamamoto T, Shen B, et al. Integrin-targeted molecular imaging of experimental abdominal aortic aneurysms by (18)F-labeled Arg-Gly-Asp positron-emission tomography. Circ Cardiovasc Imaging. 2013; 6:950–956.

43. Shi S, Orbay H, Yang Y, Graves SA, Nayak TR, Hong H, et al. PET imaging of abdominal aortic aneurysm with 64Cu-labeled anti-CD105 antibody Fab fragment. J Nucl Med. 2015; 56:927–932.

44. Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015; 6:7495.

45. Forsythe RO, Dweck MR, McBride OMB, Vesey AT, Semple SI, Shah ASV, et al. 18F-Sodium Fluoride Uptake in Abdominal Aortic Aneurysms: The SoFIA3 Study. J Am Coll Cardiol. 2018; 71:513–523.
46. Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014; 11:443–457.

47. Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002; 105:2708–2711.

48. Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013; 6:1250–1259.

49. Kim J, Choi KH, Song HC, Kim JT, Park MS, Cho KH. 18F-FDG PET/CT imaging factors that predict ischaemic stroke in cancer patients. Eur J Nucl Med Mol Imaging. 2016; 43:2228–2235.

50. Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009; 50:1611–1620.

51. Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006; 48:1825–1831.
52. Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013; 62:909–917.

53. Wu YW, Kao HL, Huang CL, Chen MF, Lin LY, Wang YC, et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging. 2012; 39:399–407.

54. Ishiwata Y, Kaneta T, Nawata S, Hino-Shishikura A, Yoshida K, Inoue T. Quantification of temporal changes in calcium score in active atherosclerotic plaque in major vessels by 18F-sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging. 2017; 44:1529–1537.

55. Broisat A, Toczek J, Dumas LS, Ahmadi M, Bacot S, Perret P, et al. 99mTc-cAbVCAM1-5 imaging is a sensitive and reproducible tool for the detection of inflamed atherosclerotic lesions in mice. J Nucl Med. 2014; 55:1678–1684.

56. Kato K, Schober O, Ikeda M, Schafers M, Ishigaki T, Kies P, et al. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur J Nucl Med Mol Imaging. 2009; 36:1622–1628.

57. Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies AH, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol. 2010; 56:653–661.

58. Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012; 2:52.

59. Rominger A, Saam T, Vogl E, Ubleis C, la Fougere C, Forster S, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med. 2010; 51:193–197.

60. Razavian M, Tavakoli S, Zhang J, Nie L, Dobrucki LW, Sinusas AJ, et al. Atherosclerosis plaque heterogeneity and response to therapy detected by in vivo molecular imaging of matrix metalloproteinase activation. J Nucl Med. 2011; 52:1795–1802.

61. Tavakoli S, Razavian M, Zhang J, Nie L, Marfatia R, Dobrucki LW, et al. Matrix metalloproteinase activation predicts amelioration of remodeling after dietary modification in injured arteries. Arterioscler Thromb Vasc Biol. 2011; 31:102–109.

62. Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using 18F-fluoromisonidazole positron emission tomographic imaging. Circ Cardiovasc Imaging. 2014; 7:312–320.

63. Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, et al. PET/CT imaging of integrin αvβ3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging. 2014; 7:178–187.

64. Paeng JC, Lee YS, Lee JS, Jeong JM, Kim KB, Chung JK, et al. Feasibility and kinetic characteristics of (68)Ga-NOTA-RGD PET for in vivo atherosclerosis imaging. Ann Nucl Med. 2013; 27:847–854.

65. Su H, Gorodny N, Gomez LF, Gangadharmath UB, Mu F, Chen G, et al. Atherosclerotic plaque uptake of a novel integrin tracer 18F-Flotegatide in a mouse model of atherosclerosis. J Nucl Cardiol. 2014; 21:553–562.

67. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990; 33:1129–1134.

68. Bleeker-Rovers CP, Bredie SJ, van der Meer JW, Corstens FH, Oyen WJ. F-18-fluorodeoxyglucose positron emission tomography in diagnosis and follow-up of patients with different types of vasculitis. Neth J Med. 2003; 61:323–329.
69. Jaruskova M, Belohlavek O. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur J Nucl Med Mol Imaging. 2006; 33:913–918.

70. Webb M, Chambers A, AL-Nahhas A, Mason JC, Maudlin L, Rahman L, et al. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging. 2004; 31:627–634.

71. Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J, et al. Role of FDG PET-CT in Takayasu arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging. 2012; 5:422–429.
72. Santhosh S, Mittal BR, Gayana S, Bhattacharya A, Sharma A, Jain S. F-18 FDG PET/CT in the evaluation of Takayasu arteritis: an experience from the tropics. J Nucl Cardiol. 2014; 21:993–1000.

73. James OG, Christensen JD, Wong TZ, Borges-Neto S, Koweek LM. Utility of FDG PET/CT in inflammatory cardiovascular disease. Radiographics. 2011; 31:1271–1286.

74. Walter MA. [(18)F]fluorodeoxyglucose PET in large vessel vasculitis. Radiol Clin North Am. 2007; 45:735–744. viii

75. Puppo C, Massollo M, Paparo F, Camellino D, Piccardo A, Shoushtari Zadeh Naseri M, et al. Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. Biomed Res Int. 2014; 2014:574248.

76. Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013; 2013:623036.
77. Salvarani C, Soriano A, Muratore F, Shoenfeld Y, Blockmans D. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017; 16:1125–1130.

78. Rehak Z, Vasina J, Ptacek J, Kazda T, Fojtik Z, Nemec P. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016; 35:398–401.

79. Sondag M, Guillot X, Verhoeven F, Blagosklonov O, Prati C, Boulahdour H, et al. Utility of 18F-fluoro-dexoxyglucose positron emission tomography for the diagnosis of polymyalgia rheumatica: a controlled study. Rheumatology (Oxford). 2016; 55:1452–1457.

80. Yamashita H, Kubota K, Takahashi Y, Minaminoto R, Morooka M, Ito K, et al. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod Rheumatol. 2012; 22:705–711.

81. Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997; 123:285–296.

83. Balink H, Bennink RJ, van Eck-Smit BL, Verberne HJ. The role of 18F-FDG PET/CT in large-vessel vasculitis: appropriateness of current classification criteria? Biomed Res Int. 2014; 2014:687608.
84. Cronin CG, Lohan DG, Blake MA, Roche C, McCarthy P, Murphy JM. Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR Am J Roentgenol. 2008; 191:423–431.

85. Vaglio A, Greco P, Versari A, Filice A, Cobelli R, Manenti L, et al. Post-treatment residual tissue in idiopathic retroperitoneal fibrosis: active residual disease or silent “scar”? A study using 18F-fluorodeoxyglucose positron emission tomography. Clin Exp Rheumatol. 2005; 23:231–234.
86. Schollhammer R, Schwartz P, Jullie ML, Pham-Ledard A, Mercie P, Fernandez P, et al. 18F-FDG PET/CT imaging of popliteal vasculitis associated with polyarteritis nodosa. Clin Nucl Med. 2017; 42:e385–e387.

87. De Geeter F, Gykiere P. (18)F-FDG PET imaging of granulomatosis with polyangiitis-Wegener's Syndrome. Hell J Nucl Med. 2016; 19:53–56.
88. Morita H, Yokoyama I, Yamada N, Uno K, Nagai R. Usefulness of 18FDG/13N-ammonia PET imaging for evaluation of the cardiac damage in Churg-Strauss syndrome. Eur J Nucl Med Mol Imaging. 2004; 31:1218.

89. Elourimi G, Soussan M, Warzocha U, Bugaud H, Dhote R, Abad S. Efficacy of tocilizumab highlighted by FDG-PET/CT in a patient with relapsing polychondritis-associated aortitis. Rheumatol Int. 2017; 37:1931–1935.

90. Kaida H, Ishii K, Hanada S, Tohda Y, Murakami T. Incidental case of relapsing polychondritis detected by 18F-FDG PET/CT. Clin Nucl Med. 2018; 43:25–27.

91. Wang J, Liu X, Pu C, Chen Y. 18F-FDG PET/CT is an ideal imaging modality for the early diagnosis of relapsing polychondritis: a case report. Medicine (Baltimore). 2017; 96:e7503.
92. Davison JM, Montilla-Soler JL, Broussard E, Wilson R, Cap A, Allen T. F-18 FDG PET-CT imaging of a mycotic aneurysm. Clin Nucl Med. 2005; 30:483–487.

93. Choi SJ, Lee JS, Cheong MH, Byun SS, Hyun IY. F-18 FDG PET/CT in the management of infected abdominal aortic aneurysm due to Salmonella. Clin Nucl Med. 2008; 33:492–495.

94. Murakami M, Morikage N, Samura M, Yamashita O, Suehiro K, Hamano K. Fluorine-18-fluorodeoxyglucose positron emission tomography-computed tomography for diagnosis of infected aortic aneurysms. Ann Vasc Surg. 2014; 28:575–578.

95. Mayer F, Aebert H, Rudert M, Konigsrainer A, Horger M, Kanz L, et al. Primary malignant sarcomas of the heart and great vessels in adult patients–a single-center experience. Oncologist. 2007; 12:1134–1142.

96. Heo SY, Park CS, Kim SJ, Park NH, Heo JH, Lee JJ. Undifferentiated pleomorphic sarcoma of the thoracic aorta: a case report. J Korean Soc Radiol. 2016; 75:304–308.

97. Sibille L, Ilonca D, Oziol E, Gandilhon P, Micheau A, Vernhet-Kovacsik H, et al. FDG PET/CT in aortic angiosarcoma. Clin Nucl Med. 2010; 35:134–137.

98. Takahashi T, Watanabe N, Wakasa M, Kajinami K, Tonami H. 18F-FDG PET/CT for detecting sarcoma of the aorta in a patient with Takayasu arteritis. Nucl Med Mol Imaging. 2016; 50:171–172.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download