Therapy-related, acute myeloid leukemia (t-AML) is a rare form of AML and is usually reported in association with chemotherapies that include alkylating agents, epipodophyllotoxin, as well as radiotherapy. t-AML generally develops years after treatments for the primary malignancy and has a poor prognosis.12 The incidence of t-AML is about 0.18% according to the United States population-based cancer registry study.3 Herein, the first case of t-AML is reported in a patient with Burkitt's lymphoma (BL) treated with multiple chemotherapeutic agents without epipodophyllotoxin.
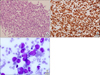
A 55-year-old male patient complaining of submental and nasopharyngeal masses was admitted to our hospital. The excised mass was positive for the leukocyte common antigens, CD20, CD79a, and Ki-67 (nearly 100%) and the diagnosis of BL was confirmed (Fig. 1A, B). The karyotype of the bone marrow (BM) cells was 46,XY. The patient was treated with eight cycles of the hyper-fractionated CVAD regimen (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), alternated with high-dose methotrexate and cytarabine. A complete response was obtained after two cycles of chemotherapy.
A complete blood count, 20 months after the last cycle, showed pancytopenia (white blood cells 6.22×109/L, hemoglobin 6.8 g/dL and platelet 24×109/L). The blood count at the time of diagnosis of BL was within normal ranges (white blood cells 7.93×109/L, hemoglobin 13.5 g/dL and platelet 261×109/L). A BM examination (Fig. 1C) revealed that about 30% blast cells (positive for CD13, CD33, CD34, HLA-DR, and myeloperoxidase, but negative for lymphoid markers). A chromosomal analysis of the BM cells showed 47,XY,+3,del(7)(q31.2q34),t(16;21)(q24;q22), and the patient was diagnosed with t-AML. Standard induction chemotherapy was administered, and one cycle of consolidation chemotherapy was added after complete remission. Finally, the patient underwent allogeneic stem cell transplantation and has been doing well for more than 2 years without recurrence of AML or BL.
t-AML is an important issue for clinicians, as it is thought to be a consequence of a mutation by prior treatment that can develop after successful chemotherapies for primary malignancies. t-AML is characterized by a latency of 5-7 years after exposure to alkylating agents or radiation therapy as well as complex karyotype and monosomy of chromosomes 5 and 7.4 Many case series in the early 1990s revealed an association between epipodophyllotoxin and t-AML in children with acute lymphoblastic leukemia. In these reports, the latent period ranged from 1 to 3 years and cytogenetic abnormalities involving 11q23 were common.1
Pancytopenia developed 20 months after completion of chemotherapy in this case of t-AML, which was first assumed to be the consequence of BL recurrence with BM involvement. However, a computed tomography scan and the BM results suggested a diagnosis of t-AML. Although the latency is relatively short, considering the complex karyotype, it seems to be associated with an alkylating agent. The only treatment option given the poor prognosis of t-AML was allogeneic stem cell transplantation.
In conclusion, t-AML can develop within 2 years following chemotherapy for BL. Allogeneic stem cell transplantation is mandatory in eligible patients considering the poor prognosis of t-AML.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download