Abstract
In this report, a case of a black meniscus with underlying ochronosis is described. Further analysis by laboratory findings showed that the patient had underlying alkaptonuria, which was previously undiagnosed. The patient's symptoms showed improvement after arthroscopic treatment.
Alkaptonuria (AKU) is a rare hereditary autosomal recessive metabolic disease that has an incidence rate of one in 250,000 to 1,000,000 live births characterized by a defect in the gene coding for homogentisate 1, 2-dioxygenase.1,2,3) As a result of this defect, the human body does not have enough of an enzyme called homogentisic acid oxidase, and homogentisic acid (HGA) accumulates. HGA is excreted in the urine, then turns dark when exposed to air due to oxidation.4) HGA has high affinity to connective tissues where it forms polymers with collagen of brown-black color. It can affect hyaline cartilages, intervertebral discs, skin and sclera. This accumulation of HGA polymers makes collagen more brittle and more vulnerable to mechanical stress. The decrease of cartilage quality causes arthropathy in large joints and the spine. Brownish black pigmentation of tissue and degenerative arthritis, accompanied with dark discoloration in urine, are called 'ochronosis'.
The diagnosis of ochronosis is usually made in a middle-aged patient (40s and older) with a triad of degenerative joint disease, ochronotic pigmentation of the sclera and skin and the characteristic urine changes.3,5,6) The skeletal system is predominantly involved, although other systems, including cardiovascular and urinary systems, may also be affected. The natural history of ochronotic arthropathy is known to be of relentless progression, sometimes rapid, that eventually leads to destruction of the joint. This disease may progress from simple AKU to alkaptonuric ochronosis and finally to ochronotic arthropathy.6,7) In this report, degenerative and complex tear of black meniscus is presented with knee arthroscopic findings.
A 78-year-old male patient with Korean nationality had suffered constant knee pain for 5 years. The patient complained pain on medial and lateral aspect of knee joint which worsened during stair walking and recurrent effusion. His symptoms were intermittently treated with knee joint aspiration and medical treatment at local clinic. Physical examination revealed effusion of knee joint. There was tenderness on medial and lateral aspect along the joint line. Range of knee motion was from 10 degrees of flexion and 120 degrees in full flexion. As a past history, he was receiving a medical treatment for benign nodule in thyroid. There was no back pain. He neither had any extraordinary medical history such as rheumatism nor possessed family history regarding AKU. Discoloration of skin, sclera, nose, and ears were not observed. There was no dark discoloration of urine.
Radiographs of knee joint demonstrated joint space narrowing and subchondral sclerosis (Fig. 1A). Magnetic resonance imaging confirmed a complex tear of the medial and lateral meniscus (Fig. 1B). Laboratory tests including the erythrocyte sedimentation rate were within normal range. Arthroscopic treatment was decided. Arthroscopic exam revealed brown pigmentation and hypertrophy of the synovium in all compartments (Fig. 2). Multiple cartilaginous and fibroid loose bodies were found. Degenerative and complex tear was found on the medial and lateral meniscus, as well as Outerbridge grade III cartilage lesion of the distal femur and proximal tibia. Discoloration of articular cartilage was not demonstrated. Debridement of the frayed tissue and synovium with partial menisectomy was performed. There was black discoloration within the substance of the medial (Fig. 3A) and lateral meniscus tear (Fig. 3B). Ligaments were intact without structural or pigmentation abnormalities. Histopathological examination of the pathologic specimen taken from the meniscus showed degenerative fibrocartilage with nonspecific brownish shards (Fig. 4).
After surgery, additional examinations for possible ochronosis were performed. The patient's urine sample produced rapid, deep brown discoloration when tested with silver nitrate and ammonium hydroxide. An elevated level of HGA (patient's level of 2.13 mmol HGA/mmol of creatinine; reference <2.00 mmol HGA/mmol of creatinine) was detected using mass spectrometry and gas chromatography which confirmed the diagnosis of AKU. Subjective symptom improvement was achieved in the early period after surgery. Four years have passed from the initial arthroscopic treatment and there is no recurrence of effusion and no loss of joint motion in recent follow-up.
The most important finding of this study is that there was symptomatic improvement of ochronotic knee joint by arthroscopic means. In the presented case, there is no recurrence of knee joint effusion and loss of motion after 4 years of initial arthroscopic treatment. It is known from previous study that arthroscopic treatment such as removal of loose bodies, debridement of frayed tissues, and removing the debris all contribute to the improvement of symptoms in these patients.8)
The diagnosis of ochronosis is usually made in middle-aged patients.3,5) Although not eminent from the presented case, the clinical symptom is usually known to begin with low back pain and stiffness. One of the characteristic roentgenographic findings are calcification of the intervertebral disc, disc space narrowing and vacuum phenomenon.3,7) Radiographic changes of the peripheral large joints appear several years later and resemble degenerative arthritis with articular space narrowing and sclerosis.4,7)
The intrasubstance deposition of pigment in the articular cartilage, menisci and synovium, and chondromalacia are the characteristic findings of ochronosis. Differential diagnosis of ochronotic arthropathy may include hemophiliac arthropathy, pigmented villonodular synovitis, and arthropathy associated with haemochromatosis etc. Diagnosis may be aided by urine analysis, detecting an elevated level of HGA in mass spectrometry and gas chromatochraphy and dark staining on alkalinisation.4,9) Ochronotic pigment deposition is known to occur in sites with high collagen content. However, extra-articular manifestations, such as pigmentation of ear auricle and sclera were not demonstrated in the presented case.
The medical treatment for ochronotic joint disease is known to be ineffective. Treatment with high dose of ascorbinic acid proved to lower the levels of HGA in excreted urine but does not prevent the occurrence of arthropathy.4,7) The aim of physical therapy and rehabilitation is to limit loss of function and progression of symptoms. Symptomatic treatment with non-steroidal anti-inflammatory drugs or surgical treatment in affected joints, such as synovectomy, arthroscopic debridement, fusion, or arthroplasty may be provided.4)
Authors experienced a case of degenerative and complex tear of black meniscus in a 78-year-old Korean male patient with AKU diagnosed and treated by arthroscopy. Knee arthroscopy is a useful diagnostic and therapeutic tool in patients with ochronotic arthropathy.
Figures and Tables
Figure 1
(A) Radiographs of the knee showing loss of joint space and subchondral sclerosis. (B) Magnetic resonance imaging with a complex tear of medial and lateral meniscus.
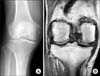
Figure 2
Arthroscopic findings. Brown pigmentation and hypertrophy of the synovium (white arrows) at medial knee compartment (A) and lateral knee compartment (B).

Figure 3
Arthroscopic findings after arthroscopic menisectomy. (A) Medial meniscus. The parenchymatous tissue of meniscus was uniformly black with sharply defined edges (arrow). (B) Lateral meniscus. Intrasubstance deposition of a grossly black substance (arrow) with yellowish-brown pigmentation of the cartilage was demonstrated.

References
1. Fernández-Cañón JM, Granadino B, Beltrán-Valero de Bernabé D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996; 14:19–24.

2. Philippot R, Coste C, Lasseur E, Tramond P, Rollier JC, Moyen B. Ochronosis or black cartilage disease. Rev Chir Orthop Reparatrice Appar Mot. 2008; 94:693–696.
3. Raaijmaakers M, Steenbrugge F, Dierickx C. Ochronosis, arthroscopy of a black knee: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008; 16:182–184.

4. Keller JM, Macaulay W, Nercessian OA, Jaffe IA. New developments in ochronosis: review of the literature. Rheumatol Int. 2005; 25:81–85.

5. Iannuccelli C, Coari G, Mastantuono M, Osimani M, Valesini G, Di Franco M. Joint and tendineous involvement in ochronosis: clinical history and imaging of a case. Clin Exp Rheumatol. 2009; 27:696–697.
6. Zhao BH, Chen BC, Shao de C, Zhang Q. Osteoarthritis? Ochronotic arthritis! A case study and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2009; 17:778–781.
7. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002; 347:2111–2121.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download