Abstract
Background
Traumatic spinal cord injury (SCI) is a tragic event that has a major impact on individuals and society as well as the healthcare system. The purpose of this study was to investigate the strength of association between surgical treatment timing and neurological improvement.
Methods
Fifty-six patients with neurological impairment due to traumatic SCI were included in this study. From January 2013 to June 2017, all their medical records were reviewed. Initially, to identify the factors affecting the recovery of neurological deficit after an acute SCI, we performed univariate logistic regression analyses for various variables. Then, we performed a multivariate logistic regression analysis for variables that showed a p-value of < 0.2 in the univariate analyses. The Hosmer-Lemeshow test was used to determine the goodness of fit for the multivariate logistic regression model.
Results
In the univariate analysis on the strength of associations between various factors and neurological improvement, the following factors had a p-value of < 0.2: surgical timing (early, < 8 hours; late, 8–24 hours; p = 0.033), completeness of SCI (complete/incomplete; p = 0.033), and smoking (p = 0.095). In the multivariate analysis, only two variables were significant: surgical timing (odds ratio [OR], 0.128; p = 0.004) and completeness of SCI (OR, 9.611; p = 0.009).
Conclusions
Early surgical decompression within 8 hours after traumatic SCI appeared to improve neurological recovery. Furthermore, incomplete SCI was more closely related to favorable neurological improvement than complete SCI. Therefore, we recommend early decompression as an effective treatment for traumatic SCI.
Traumatic spinal cord injury (SCI) with neurological deficit is a tragic event, causing a major burden on the individual and society. Its worldwide prevalence is approximately 750 per million and the annual incidence appears to be increasing.12) Thus, not only prevention of SCI but also treatment strategies for neurological recovery are critical issues for spine surgeons, spine associations, and national health institutions.
Many experimental studies as well as clinical research series on traumatic SCI addressed the concepts of primary and secondary injuries and how they relate to the mechanism of neurological injury. The primary injury is caused by direct trauma mainly to the vertebrae, such as a cord contusion or compression. This initiates a series of downstream cellular response, leading to a secondary injury that, in the case of SCI, involves a cascade of pathophysiological events triggered by the primary injury; the continuing compression or displacement can cause neurological impairments by blood flow interruptions, alterations of electrolytes, neurotransmitter accumulations, the release of oxygen free radicals, inflammations, and edema formations.34) If prevention of the primary injury is not possible, then minimizing the secondary injury is the only available therapeutic target for individuals with a traumatic SCI.5)
The timing of surgical decompression is an important clinical issue. Although the optimal timing remains controversial, decompression of the spinal cord, stabilization of the vertebra, and maintenance of blood perfusion are well established as critical factors in generating optimal outcomes in traumatic SCI. Despite many studies reporting improvement in neurological outcomes through early surgical decompression in traumatic SCI, there is no consensus on the definition of “early decompression.”67) While reviewing the literature, we noticed that the definition of early timing varied from 4 hours to 4 days, but the trend since 2010 has been to perform decompression within 24 hours of the injury.8) Few studies used a cut-off time of 24 hours to compare early and late groups. However, many experimental animal models and clinical investigations indicated that the first 8 hours are the optimal therapeutic window for early spinal cord decompression.910)
Based on such findings on the time window of surgical interventions for acute traumatic SCI, we aimed in the present study to evaluate whether an early surgical decompression (< 8 hours) is more effective than a late procedure (8–24 hours) with respect to the clinical outcomes and to investigate the strength of associations between various factors and neurological improvement. We hypothesized that the neurological improvement would be superior in the early surgical decompression group compared to the late decompression group.
We conducted this study in compliance with the principals of the Declaration of Helsinki. The protocol of study was approved by institutional review board of Gyeongsang National University Hospital (GNUH IRB 2016-07-002-001). Written informed consents were obtained. From January 2013 to June 2017, the medical records of all patients who had sustained an SCI due to an acute traumatic injury were reviewed and analyzed. The inclusion criteria were a traumatic SCI with a neurological deficit (American Spinal Injury Association [ASIA] impairment scale [AIS] A–D), a lesion between C1 and L2, patients with no spinal shock or patients who were improved from spinal shock, a stable medical condition, patients who underwent surgical spinal cord decompression within 24 hours of the injury, follow-up for at least 6 months, only adult patients, and no other systemic or life-threatening injuries. The exclusion criteria were the absence of neurological deficits, no surgical procedure, decompression > 24 hours post-injury, a cognitive impairment to the extent of rendering neurological assessment impossible, ossification of the posterior longitudinal ligament, an unstable medical condition, and the existence of another systemic or life-threatening injury. The inclusion criteria were met by 57 patients.
According to the surgical decompression time, patients were divided into the early (< 8 hours) and late (8–24 hours) surgical decompression groups. Based on the AIS, the SCI was classified as complete (A) or incomplete (B–D) SCI. Various clinical parameters were recorded, including the subjects' sex, age, smoking habits, comorbidities, mechanism of injury, accompanying injuries, fracture characteristics, surgical interventions, the number of fused segments, the length of intensive care unit (ICU) stay, the period of hospitalization, the duration of mechanical ventilation, and the follow-up period. We also documented whether the patient was transferred from another hospital, as well as the presence of perioperative complications during hospital stay.
Neurological manifestations were assessed by a fellowship-trained orthopedic surgeon (DYL) using standards established by the ASIA. We recorded the neurological level of impairment, the preoperative AIS class, the postoperative AIS class, and improvement in AIS class. The postoperative AIS class was evaluated not earlier than 6 months after surgery. To quantify the degree of neurological improvement, the AIS classes (A–E) were converted to numerical values, from 1 (class A, worst grade) to 5 (class E, best grade).
Data are presented as numbers, percentages, and means and standard deviations. The associations between various factors and the neurological improvement were evaluated by calculating the odds ratios (ORs) and 95% confidence intervals [CIs], using logistic regression analysis. First, we performed univariate logistic regression analyses for all variables; then, we performed a multivariate logistic regression analysis, using only the p-value < 0.2 variables from the univariate analyses. The Hosmer-Lemeshow test was used to determine the goodness of fit for the multivariate logistic regression model. All statistical analyses were performed using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) with significance set at p < 0.05, except for the Hosmer-Lemeshow test where the significance was set at p > 0.05.
During the 5-year study period, 57 subjects who met the inclusion criteria were evaluated, but one patient died from pulmonary failure, leaving 56 patients available for the final analysis. The most frequent injury types were falls from a height (n = 26, 46.4%) and traffic accidents (n = 18, 32.1%). The most common level of impairment was the cervical spine (n = 18, 32.1%), and 17 patients (30.4%) had accompanying injuries. Spinal fusion was the most frequently performed surgery; for patients who underwent fusion alone or anterior cervical discectomy and fusion, the mean number of fused segments was 2.8 ± 1.3. The identified perioperative complications included pneumonia, pressure ulcers, deep vein thrombosis, postoperative wound infections, cardiovascular complications, and gastrointestinal problems, with pressure ulcers being the most common complication (Table 1).
The evaluation of the strength of association between various factors and the neurological improvement yielded the following results. In the univariate analyses, the factors that had a p-value of < 0.2 were surgical timing, completeness of SCI, and smoking. In the multivariate analysis, the two variables, surgical timing (OR, 0.128; 95% CI, 0.031 to 0.521; p = 0.004) and completeness of SCI (OR, 9.611; 95% CI, 1.748 to 52.848; p = 0.009), had a statistically significant association with neurological recovery, as shown in Table 2. These results mean early surgical decompression and incomplete SCI are closely associated with favorable neurological improvement after acute SCI. The p-value of the Hosmer-Lemeshow test was 0.581, indicating a good fit.
The preoperative neurological manifestation was a complete SCI (AIS class A) in 15 patients and an incomplete SCI in 41 patients. After the operation, 12 patients continued to have a complete SCI, but three improved; thus, the number of subjects with an incomplete SCI became 44. The distributions of AIS grades at admission and discharge were not significantly different between early and late decompression groups (p > 0.05). Improvement in the AIS grade was evidenced in 16 patients (61.5%) from the early decompression group and in nine (30.0%) from the late decompression group. The degree of AIS grade improvement was 0.65 ± 0.56 in the early decompression group and 0.30 ± 0.47 in the late decompression group (p = 0.018) (Tables 3 and 4). In terms of the completeness of SCI, there was no significant difference in the degree of recovery of neurological damage between early and late decompression groups in the complete SCI group (p = 0.792); in the incomplete SCI group, the degree of recovery of neurological damage was significantly good (p = 0.002).
We performed a single-institution, retrospective cohort study to evaluate the effect of surgical decompression timing within the first 24 hours after traumatic SCI. In accordance with our hypothesis, we found that neurological improvement, which is one of the most salient clinical manifestations, was superior when surgical decompression was performed within the first 8 hours compared to 8 to 24 hours after injury. Therefore, to achieve higher recovery rates, decompression should be performed within 8 hours after the initial trauma. Furthermore, spine surgery is a major surgical procedure that has a high mortality. In our study, one patient died of pulmonary failure even though all medically unstable patients had been excluded from the study. Therefore, considering the high possibility of death from complications after surgery, it is important to maintain stable cognitive and medical conditions through comprehensive evaluation before surgery.
Various outcomes, including the duration of ICU stay, length of hospitalization, the time for mechanical ventilation, and perioperative complications, were not significantly different between the early and late surgery groups. By contrast, the meta-analysis by Liu et al.11) showed an association between early surgical decompression for traumatic SCI and shorter hospital stay as well as fewer perioperative complications. This discrepancy with our results may be attributed to various factors. First, the present study had a relatively small sample size and slightly different inclusion criteria, and the heterogeneous characteristics of the subjects between the two studies might have caused different results. Second, some reports suggest that the perioperative complication rate increases when an operation is performed too early after injury;1213) therefore, we only included patients who were considered medically stable for surgery, which is why the early group did not exhibit an excessive perioperative complication rate. Third, the research by Carreon and Dimar14) and Liu et al.11) set the post-injury cut-off time between early versus late surgery at 24 hours and 72 hours, respectively. Indeed, there is no consensus on the criteria for differentiating early and late surgery, although during the past 5 years, the most commonly used cut-off time has been 24 hours. However, a few studies including ours have used the 8 hour cut-off time.151617) Our results only reflect the effects of early surgical decompression performed within 8 hours after acute SCI. Therefore, for more definite conclusions regarding the effect of timing of surgery on various clinical outcomes, further studies that compare the outcomes of surgical decompression performed during the first 8 hours and 8–24 hours after acute traumatic SCI are required.
Not only the surgical timing for acute SCI but also the completeness of SCI is critical to prognosis. While many studies have shown improvement in neurological outcomes in patients with incomplete SCI, the effect of surgery for complete SCI is believed to be poor. In addition, some studies suggested that surgical intervention provided no benefits regarding neurological recovery in complete SCI.1819) Pollard and Apple20) suggested that the most important prognostic variable relating to neurological recovery in a patient with a SCI is the completeness of the lesion. In other words, an incomplete spinal cord lesion has a more favorable prognosis for neurological recovery. These results are in accordance with the present study's outcomes; thus, we believe early surgical decompression and incomplete SCI are closely related to favorable neurological recovery.
Although transfer from another hospital was not a risk factor for neurological improvement, the transfer rate was significantly higher in the late decompression group. It can be inferred that one of the main reasons for the late surgical decompression was the time needed for transfer from a different hospital. If patients with traumatic SCI first visit a small clinic with no spine specialists, they have to be transferred to a specialized spinal center, which can conceivably impede early surgery. For this reason, we strongly recommend immediate transfer of traumatic SCI patients from the injury location to a specialized spine institution.
The primary mechanism of traumatic SCI is the traumatic event itself, which causes an initial cord lesion resulting from physical injury caused by displacement or compression from surrounding spinal structures.21) However, currently there is no treatment that can reduce the effects of the primary SCI mechanism. If it cannot be prevented, minimizing the secondary mechanism of injury represents the only therapeutic option for patients with traumatic SCI. The theoretical concepts of primary and secondary mechanisms establish the basis of spinal decompression surgery for traumatic SCI, which is supported by our study where beneficial effects were observed with early surgical decompression during the first 8 hours following an injury. Previous preclinical studies also reported that early surgical decompression during the first 6–8 hours after SCI enhanced recovery.2223) Furthermore, the National Acute Spinal Cord Injury Study 2 and 3 trials suggested up to 8 hours as the optimal therapeutic window for SCI.910) Hakalo and Wronski24) also suggested that early spinal cord decompression within these 8 hours is optimal for neurological recovery, based on their analysis of SCI patients' clinical data. Their findings support the need for early surgical decompression and highlight the demand for an improved efficiency of transfers to shorten the arrival time at a specialized spine center. Two previous randomized controlled trials comparing early (< 8 hours) versus late (> 8 hours) surgery for traumatic SCI also found that early decompression was associated with superior neurological outcomes.1617) In contrast, Pointillart et al.25) could not identify any significant difference in neurological recovery after traumatic SCI when surgical decompression was performed < 8 hours or 8–24 hours after injury; however, the primary focus of their study was not on the effect of surgical intervention timing but on the effectiveness of pharmacological therapy, so it might be difficult to compare their results with those of ours. Overall, previous study results and our findings can be interpreted as supporting early surgery for acute traumatic SCI since early surgical decompression is likely associated with the recovery of neurological deficit.
The current study has some limitations. First, it is a retrospective study. For high-quality medical research, large-scale randomized controlled trials are preferred. However, we believe that delaying early surgery for the purpose of randomization would be unethical. Nevertheless, a late surgery would be unavoidable in some cases due to transfer from the traumatic incident site to a specialized spine institution, and patients from these cases can be used for comparison in future studies without violating ethical principles. Second, the study sample was relatively small. Third, the effects of medications were not considered. A number of drugs are used to facilitate neurological recovery of patients with traumatic SCI, and the only medication that our subjects used was mega-dose steroids. Since such medicinal therapy is not considered very effective, its impact as a confounder may have been minimal. Fourth, we did not take into account the difference in the level of lesion that may have different spinal cord recovery mechanisms. For instance, since the thoracolumbar junction (T11–L2) levels contain both upper and lower motor neurons, the healing process could be different from that at other levels.26) Therefore, large-scale prospective studies are recommended to overcome these limitations and confirm the effectiveness of early surgical decompression after acute traumatic SCI.
In conclusion, our study demonstrated that early surgical decompression performed within 8 hours after traumatic SCI could improve neurological recovery. Furthermore, incomplete SCI was more closely related to favorable neurological improvement than complete injury. Therefore, we recommend early decompression as an effective treatment for traumatic SCI.
References
1. Wyndaele M, Wyndaele JJ. Incidnce, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006; 44(9):523–529. PMID: 16389270.
2. Kim YH, Ha KY, Kim SI. Spinal cord injury and related clinical trials. Clin Orthop Surg. 2017; 9(1):1–9. PMID: 28261421.

3. Maikos JT, Shreiber DI. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma. 2007; 24(3):492–507. PMID: 17402855.

4. Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+)-H+ exchanger, and the Na(+)-Ca2+ exchanger. J Neurosci. 1996; 16(2):545–552. PMID: 8551338.

5. Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011; 28(8):1371–1399. PMID: 20001726.

6. Umerani MS, Abbas A, Sharif S. Clinical outcome in patients with early versus delayed decompression in cervical spine trauma. Asian Spine J. 2014; 8(4):427–434. PMID: 25187859.

7. Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One. 2012; 7(2):e32037. PMID: 22384132.

8. El Tecle NE, Dahdaleh NS, Hitchon PW. Timing of surgery in spinal cord injury. Spine (Phila Pa 1976). 2016; 41(16):E995–E1004. PMID: 26909843.

9. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405–1411. PMID: 2278545.
10. Bracken MB, Shepard MJ, Holford TR, et al. National Acute Spinal Cord Injury Study. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. JAMA. 1997; 277(20):1597–1604. PMID: 9168289.
11. Liu JM, Long XH, Zhou Y, Peng HW, Liu ZL, Huang SH. Incidnce, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? A meta-analysis. World Neurosurg. 2016; 87:124–131. PMID: 26724625.
12. Clohisy JC, Akbarnia BA, Bucholz RD, Burkus JK, Backer RJ. Neurologic recovery associated with anterior decompression of spine fractures at the thoracolumbar junction (T12-L1). Spine (Phila Pa 1976). 1992; 17(8 Suppl):S325–S330. PMID: 1523520.

13. Krengel WF 3rd, Anderson PA, Henley MB. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine (Phila Pa 1976). 1993; 18(14):2080–2087. PMID: 8272964.

14. Carreon LY, Dimar JR. Early versus late stabilization of spine injuries: a systematic review. Spine (Phila Pa 1976). 2011; 36(11):E727–E733. PMID: 21270685.
15. Jug M, Kejzar N, Vesel M, et al. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: a single center experience. J Neurotrauma. 2015; 32(18):1385–1392. PMID: 25658291.

16. Chen Q, Li F, Fang Z, et al. Timing of surgical decompression for acute traumatic cervical spinal cord injury: a multicenter study. Neurosurg Q. 2012; 22(1):61–68.
17. Cengiz SL, Kalkan E, Bayir A, Ilik K, Basefer A. Timing of thoracolomber spine stabilization in trauma patients; impact on neurological outcome and clinical course: a real prospective (RCT) randomized controlled study. Arch Orthop Trauma Surg. 2008; 128(9):959–966. PMID: 18040702.

18. Zariffa J, Curt A, Steeves JD. Functional motor preservation below the level of injury in subjects with American Spinal Injury Association Impairment Scale grade A spinal cord injuries. Arch Phys Med Rehabil. 2012; 93(5):905–907. PMID: 22360976.

19. Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007; 45(3):190–205. PMID: 17179973.

20. Pollard ME, Apple DF. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine (Phila Pa 1976). 2003; 28(1):33–39. PMID: 12544952.

21. Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004; 21(4):429–440. PMID: 15115592.

22. Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury: recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995; 77(7):1042–1049. PMID: 7608226.

23. Fehlings MG, Tator CH. An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999; 91(1 Suppl):1–11.

24. Hakało J, Wronski J. Importance of early operative decompression of spinal cord after cervical spine injuries. Neurol Neurochir Pol. 2004; 38(3):183–188. PMID: 15354230.
25. Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000; 38(2):71–76. PMID: 10762178.

26. Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006; 59(5):957–982. PMID: 17143232.
Table 1
Characteristics and Outcomes of Patients with Traumatic Spinal Cord Injury
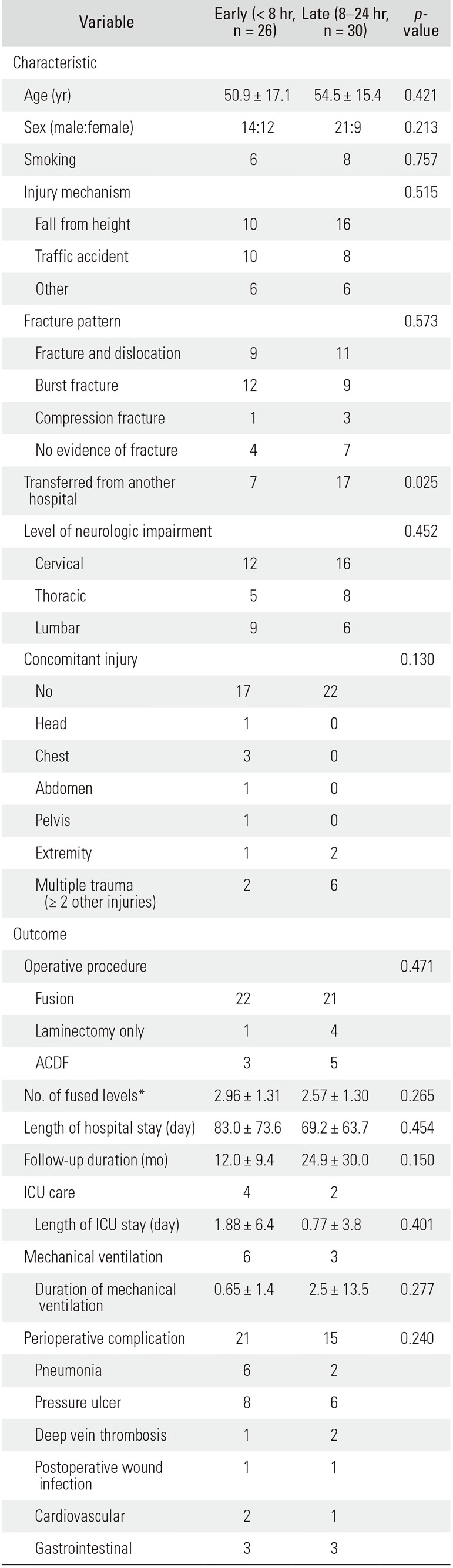
Values are presented as mean ± standard deviation or number. According to Denis classification, fracture patterns were categorized.
ACDF: anterior cervical discectomy and fusion, ICU: intensive care unit.
*Number of segments fused in those patients who underwent spinal fusion with instrumentation or ACDF.
Table 2
Strengths of Associations between Various Factors and the Improvement of Neurologic Deficit in the Univariate and Multivariate Analyses
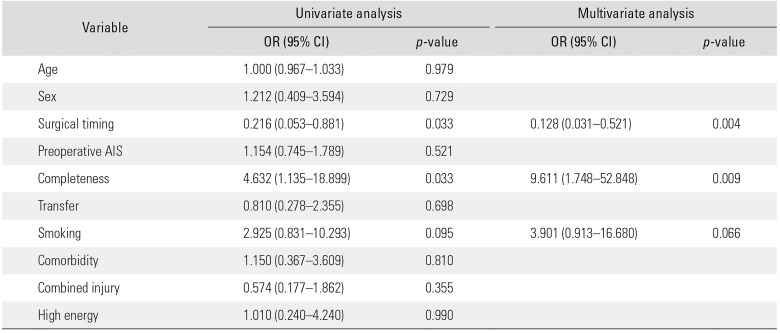
Table 3
Neurologic Improvement Grades of Patients with Acute Traumatic Spinal Cord Injury*
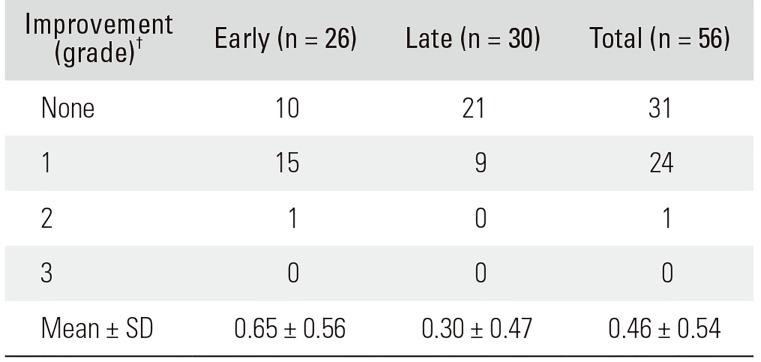
| Improvement (grade)† | Early (n = 26) | Late (n = 30) | Total (n = 56) |
|---|---|---|---|
| None | 10 | 21 | 31 |
| 1 | 15 | 9 | 24 |
| 2 | 1 | 0 | 1 |
| 3 | 0 | 0 | 0 |
| Mean ± SD | 0.65 ± 0.56 | 0.30 ± 0.47 | 0.46 ± 0.54 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download