Abstract
Background
This study evaluated the effects of Beraprost sodium (Berasil) on subjective leg symptoms in patients with peripheral arterial disease caused by diabetes mellitus.
Methods
Ninety-four diabetic patients with peripheral arterial disease were treated with Beraprost in a fixed-dose, prospective, multicenter, cohort study. Beraprost (40 µg) was administered orally 3 times daily (120 µg/day) for 12 weeks. We developed a new disease-specific symptom questionnaire, which evaluated the effect of peripheral arterial disease on leg discomfort in daily life and assessed therapeutic responses to treatment. Patients were asked for their subjective assessment of symptoms on a written questionnaire before treatment and after 12 weeks of therapy.
Results
There was significant improvement in all estimated subjective symptoms (burning, coldness, edema, exertional pain, stabbing, and paresthesias) in the lower extremities at 12 weeks (p < 0.001). There were 18 patients with neuropathy in whom significant improvement was noted for 6 subjective symptoms at 12 weeks (p < 0.05). Adverse events considered to be drug-related were observed in 4 patients (4.3%), all of which were mild and resolved with discontinuation of the medication.
Peripheral arterial disease (PAD) is a disorder characterized by narrowing or occlusion of the arteries that result in a lack of adequate arterial blood to the limbs. Approximately 120 to 140 million people suffer from diabetes mellitus (DM) worldwide and patients with diabetes more commonly develop symptomatic PAD.1) The Framingham Study showed that the presence of diabetes increased the risk of developing PAD by 3.5- and 8.6-fold among men and women, respectively.2) PAD in DM is more severe in extent and progresses more rapidly than in non-diabetic patients.3)
The goals in treatment of the patients with PAD are the improvement in symptoms and prevention of cardiovascular events. Many agents, including antiplatelet drugs and vasodilators, have been tried for pain relief, although their effectiveness remains the subject of debate. Prostaglandin I2 (PGI2), a naturally occurring prostanoid, promotes vasodilatation,4,5) inhibits platelet aggregation,4,5) and suppresses smooth muscle proliferation.6) Beraprost sodium (Berasil, Astellas Pharma Korea, Seoul, Korea) is an orally active PGI2 analogue with antiplatelet and vasodilating properties.7,8) The Beraprost et Claudication Intermittent study (BERCI-2), a double-blind, randomized, and multicenter controlled trial in France and Italy, demonstrated that both maximum walking distance and pain-free walking distance were increased significantly in patients receiving Beraprost.9) However, only a few patients with non-insulin dependent DM were included and patients with insulin dependent DM were excluded in their study. Most of the previous studies regarding the effects of Beraprost on PAD have not addressed diabetic patients9,10) and only a few studies have been performed in regard to the treatment of Beraprost in diabetic patients with PAD.11)
Intermittent claudication has been considered the most characteristic manifestation of PAD.12) The best way of evaluating ambulatory function and assessing the severity of intermittent claudication in patients with PAD is by measuring maximal walking distance and time recorded during a treadmill exercise test.13) However, in community-based epidemiologic studies, many people with PAD have symptoms other than classic intermittent claudication.14-16) Therefore, a treadmill exercise test cannot always accurately reflect the symptoms patients experienced in their daily life. Questionnaires are frequently used as a tool to assess pain syndromes. Current pain questionnaires, such as the McGill Pain Questionnaire17) and the Neuropathic Pain Scale,18) are not specific enough for the assessment of the different symptoms of diabetic patients with PAD. To our knowledge, there have been no studies that have evaluated the intensity and quality of subjective symptoms or have assessed the ability of Beraprost to improve these symptoms in diabetic patients with PAD. We developed a new disease-specific symptom questionnaire, which evaluated the effects of PAD on leg discomfort in daily life in order to assess therapeutic responses to Beraprost. The present study aimed to evaluate the effects of Beraprost for improving subjective leg symptoms using the newly devised, disease-specific symptom questionnaire in patients with peripheral arterial diseases and diabetes.
The current investigation was a prospective, multicenter, cohort study conducted at third referral hospitals in South Korea, between December 2009 and October 2010. Patients who met the following criteria were included in this study: 1) between 18 and 90 years of age; 2) an established diagnosis of DM; 3) lower limb discomfort for longer than six months; 4) a diagnosis of PAD established by the absence of one or more foot pulses of the involved foot or an ankle brachial pressure index (ABI) less than 0.9. Patients were excluded from the study if they had critical limb ischemia, defined as the presence of rest pain requiring analgesics for a duration of more than 2 weeks, the presence of non-healing ischemic ulcer, or gangrene; a stroke or myocardial infraction within the last 3 months; a tendency towards hemorrhage; severe liver or kidney disease; having undergone endovascular intervention of the coronary artery or a peripheral artery; or having undergone operative revascularization within the last 3 months. With these strict inclusion and exclusion criteria, 100 diabetic patients with PAD were enrolled in this study, 6 of whom were excluded because they chose to discontinue the medication. All patients who were eligible for the study agreed to participate and signed a written informed consent. This study was performed according to the Declaration of Helsinki and was approved by the respective local Institutional Review Boards from the institutions participating in the study.
Patients were administered oral Beraprost (40 µg) 3 times daily (120 µg/day) for 12 weeks. Use of other drugs with significant effects on peripheral vessels, hemostasis, or platelet function during the study was prohibited. Among these included pentoxifylline, heparin, warfarin, aspirin, persantine, ticlopidine, and prostaglandin E1 analogs. All other medical care regimens were continued throughout the course of the study in all the patients.
The following safety assessments were made before treatment, as well as 6 weeks and 12 weeks thereafter, or at drug discontinuation: serum chemistry, hematology, electrocardiogram, vital signs, concomitant medication changes, and adverse events.
First, a list of descriptors was chosen on the basis of our experience and analysis of the literature. Following discussions and approval of content validity by a panel of 4 Korean diabetic foot experts, we included 8 descriptors (burning, coldness, edema, exertional pain, throbbing, shooting, stabbing, and paresthesias) in the initial version of the questionnaire. A pilot study was performed in all patients who participated in this study at their first visit in order to verify the face validity of the questionnaire. The patients were asked to complete the questionnaire and to rate each descriptor for clarity in wording, understanding, and relevance to their current symptoms. After the pilot study, the "throbbing" descriptor was excluded because it was considered irrelevant by a majority of patients. In addition, the "shooting" descriptor was excluded because of very low prevalence (< 30% of the patients reported the symptom) as compared to the other descriptors. Thus, the final version of the disease-specific symptom questionnaire included 6 descriptors: burning, coldness, edema, exertional pain, stabbing, and paresthesias. Each subjective symptom was ranked from 0 to 4, according to the severity of the symptoms felt by the patient; 0 reflected asymptomatic status and 4 reflected the greatest severity. Patients were asked for their subjective assessment of their symptoms in the written questionnaire before treatment and at 12 weeks. In addition to the assessment of subjective symptoms, patients were evaluated serially for new complaints, changes in symptoms, and functional status.
Statistical analyses were consequently performed with the SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Differences between pretreatment and the last follow-up were analyzed by the Wilcoxon matched-paired signed-ranks test. A value of p < 0.05 was considered statistically significant.
Among 94 patients included in the evaluation, 90 patients completed the study. Four patients failed to qualify for the study because of adverse reactions to Beraprost, the study medication. Table 1 shows the patients' demographics. The mean age was 63.5 years (range, 30 to 83 years) and the mean duration of DM was 9.3 years (range, 1 to 30 years). Diabetic retinopathy, nephropathy, and neuropathy were present in 8 patients (8.9%), 11 patients (12.2%), 18 patients (20.0%), respectively, some of whom had more than one complication. Comorbidities, including hypertension, hyperlipidemia, and previous myocardial infarction were noted in 57 patients. Ten patients were either current smokers or had a history of smoking within the past 2 years. No specific counseling on diet, smoking cessation or exercise was offered during the study period. These factors were not specifically controlled.
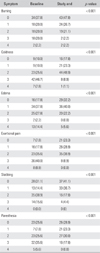
Table 2 lists the scores for each symptom at baseline and after Beraprost treatment. The most commonly reported symptom was exertional pain (83 patients, 92.2%). Of these patients, 69.9% reported improvement in their symptoms. Of 81 patients with coldness, 59 patients (72.8%) showed improvement in their symptoms, 21 patients (25.9%) remained the same, and one patient (1.2%) worsened. Coldness was the most improved among the subjective symptoms with taking the medication. Seventy-four patients had edema, and 39 patients (52.7%) showed improvement in this symptom. Stabbing, burning, and paresthesias were observed in 61, 56, and 67 patients, respectively, and 65.6%, 64.3%, and 55.2% of them showed improvement in the respective symptoms. Significant improvement in all estimated subjective symptoms (coldness, edema, exertional pain, stabbing, burning, and paresthesias) in the lower extremities was reported at 12 weeks (p < 0.001).
There were 18 patients with neuropathy that was determined by an absent protective threshold using a 5.07 (10 g) Semmes-Weinstein monofilament. With the numbers available, these patients that had accompanying neuropathy showed significant improvement in all 6 subjective symptoms at 12 weeks (p < 0.05) (Table 3).
There were no reports of critical cardiovascular events, including myocardial infarction, transient ischemic attack or ischemic gangrene, during the treatment period with Beraprost. Adverse reactions considered to be causally related to Beraprost occurred in 4 of the 94 patients (4.3%), and all of these patients discontinued treatment prematurely. Headache was the most common complaint, occurring in 2 patients (2.1%). Insomnia (one patient) and dyspepsia (one patient) were reported as well. All adverse events were mild and resolved after discontinuing the medication. No significant changes were noted in the safety assessment thereof.
Treatment of PAD can be considered to take part in 3 stages: lifestyle and risk factor modification, pharmacotherapies, and revascularization. Treatment of the patient's lower extremity symptoms should be chosen on the basis of the severity of the symptoms. Pharmacotherapy includes vasodilating agents, calcium channel blockers, anticoagulants, antiplatelets, and prostaglandin analogs. A revascularization procedure, including surgical or endovascular intervention, is usually reserved for patients with disabling claudication that affects their quality of life, after medical therapy has failed to improve symptoms and critical limb ischemia symptoms.
Various medications have been investigated in patients with PAD. Currently, 2 agents are available in the United States for the symptomatic treatment of PAD: pentoxifylline and cilostazol. Both drugs have shown to increase pain-free walking time and total distance walked, although the data on pentoxifylline is conflicting.19,20) In comparison with the placebo, cilostazol improved the distance that patients with intermittent claudication were able to walk during standardized treadmill testing.21,22) However, its use is contraindicated in patients with heart failure, and a sizable minority discontinues treatment due to gastrointestinal upset or palpitations.
Prostaglandins have also shown clinical benefit in reducing the symptoms of intermittent claudication and significant increase in walking distance.23,24) PGI2 which is synthesized in vascular endothelial and smooth muscle cells, shows antiplatelet action and vasodilating action.4,5) Beraprost sodium is a stable, orally active PGI2 analogue, launched in Japan in 1992, and is currently used in several countries to treat ischemic symptoms in chronic arterial occlusion. Beraprost has favorable effects on maximal treadmill walking distance and quality of life, while reducing the incidence of critical cardiovascular events in patients with intermittent claudication at 6 months.9) However, another trial of Beraprost that involved 897 patients in the United States showed no significant improvement in maximal walking distance.10) Although the two trials were well designed, only a few patients with DM were included in these studies. For that reason, it is necessary to test the efficacy of Beraprost treatment in patients with DM specifically.
PAD treatment is primarily directed at palliating symptoms and improving patients' ambulatory function. Intermittent claudication has been considered the most characteristic manifestation of PAD.12) However, in community-based epidemiologic studies, many people with PAD have symptoms other than classic intermittent claudication.14-16) Significant improvement of pain-free walking distance or maximum walking distance has been demonstrated. However, if the patients' subjective symptoms assessment is not changed, there is no reason to consider the effect to be beneficial. Thus, we developed a new disease-specific symptom questionnaire, which evaluated the effects of PAD on leg discomfort in daily life, to assess therapeutic responses.
In this study, 6 subjective symptoms were evaluated as parameters of the drug effect. Oral administration of Beraprost over a period of 12 weeks resulted in significant improvement in all measures of subjective symptoms in diabetic patients with PAD (p < 0.001). This suggests that treatment with Beraprost can improve a patient's quality of life. The most commonly reported symptom was exertional pain (83 patients, 92.2%). Of these patients, 69.9% reported improvement in their symptoms. Coldness was the next commonly reported subjective symptom, occurring in 90.0% of the enrolled patients, and 72.8% of these patients reported improvement in this symptom. Coldness was the most improved among the subjective symptoms.
Neuropathies developing in patients with diabetes are known to be heterogeneous in respect to their symptoms, pathologic alterations, and pattern of neurologic involvement. Chronic neuropathic pain is present in 13% to 26% of diabetic patients.25-28) The symptom in diabetic painful neuropathy was most often described by the patients as "burning/hot," "electric," sharp," "achy," and "tingling".29) These symptoms are similar to those observed in PAD patients. Therefore, when PAD patients accompanied with neuropathy are treated with Beraprost, it is difficult to predict the outcome. In this study, we observed improvement in all of the 6 symptom parameters in patients accompanied with neuropathy. Therefore, if PAD is prevalent in patients suspected to have diabetic neuropathy, we may expect improvement in some symptoms by treating PAD.
One study of Beraprost demonstrated that Beraprost increased blood flow significantly to the skin of the feet in patients with type 2 diabetes.30) Consistent with an increase in ABI, significant improvement in coldness of the limb, numbness, and paralysis was observed in diabetic patients with PAD after treatment with Beraprost.11) In this study, a 12-week treatment with Beraprost significantly improved edema (39 of 74 patients, 52.7%) and coldness (59 of 83 patients, 72.8%). All of the above suggests that, in treatment of diabetic circulatory disorders, Beraprost dilates peripheral vessels and increase blood flow to the skin, resulting in the improvement of various symptoms.
In the current study, adverse events considered by the investigators to be drug-related were observed in 4 patients (4.3%), including headache (2 patients, 2.1%), insomnia (one patient, 1.1%), and dyspepsia (one patient, 1.1%). All adverse events were mild and resolved with discontinuation of the medication. The incidence of adverse events in our study was relatively low compared to that in previous studies.9-11) This is probably because the period of medication was relatively short in our study.
The limitations of this study include the uncontrolled and nonrandomized design, as well as the relatively small sample size. In addition, the lack of placebo control limits the statistical power of the data. Also, there was a limited ability to perform analyses of a subgroup with neuropathy because sample size could be decreased further. In the present study, various antipyretic analgesics, tricyclic antidepressants, the anticonvulsants gabapentin and pregabalin, as well as serotonin and norepinephrine reuptake inhibitors were used concomitantly with Beraprost. Therefore, to verify the contributory effect of Beraprost treatment, separate analyses should have been done before and after Beraprost treatment for groups using and not using each concomitant drug. This study was relatively short in duration. However, there was an effort to minimize the effects of other variables. No changes in activity or risk factor modification were recommended during the trial. By minimizing the biases coming from these factors, these similarities lent statistical power to our results. Finally, objective findings such as ABI and transcutaneous oxygen saturation were not measured to assess the efficacy of Beraprost. Instead, this study focused on the effect of Beraprost on subjective leg symptoms in diabetic patients with PAD. In future prospective randomized studies, evaluation of ABI or transcutaneous oxygen saturation are required to determine whether objective findings indeed improve.
Diabetic patients with PAD may have various symptoms that are generally reproducible during daily activities, resulting in a negative impact on quality of life. Beraprost is an agent with pharmacological actions unlike other drugs in respect to the treatment of PAD. This trial showed statistically significant improvement of subjective symptoms in diabetic patients with PAD after 12 weeks of therapy with Beraprost, even without specific risk-factor modification and a directed exercise program. Beraprost may present a new option for the treatment of various symptoms in diabetic patients with PAD. Further studies are needed with a larger sample size and a placebo control to confirm our results.
Figures and Tables
References
1. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003. 26(12):3333–3341.
2. Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985. 33(1):13–18.

3. Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001. 24(8):1433–1437.
5. Friedman R, Mears JG, Barst RJ. Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation. 1997. 96(9):2782–2784.

6. Willis AL, Smith DL, Vigo C, Kluge AF. Effects of prostacyclin and orally active stable mimetic agent RS-93427-007 on basic mechanisms of atherogenesis. Lancet. 1986. 2(8508):682–683.

7. Demolis JL, Robert A, Mouren M, Funck-Brentano C, Jaillon P. Pharmacokinetics and platelet antiaggregating effects of beraprost, an oral stable prostacyclin analogue, in healthy volunteers. J Cardiovasc Pharmacol. 1993. 22(5):711–716.

8. Nony P, Ffrench P, Girard P, et al. Platelet-aggregation inhibition and hemodynamic effects of beraprost sodium, a new oral prostacyclin derivative: a study in healthy male subjects. Can J Physiol Pharmacol. 1996. 74(8):887–893.

9. Lièvre M, Morand S, Besse B, Fiessinger JN, Boissel JP. Beraprost et Claudication Intermittente (BERCI) Research Group. Oral Beraprost sodium, a prostaglandin I(2) analogue, for intermittent claudication: a double-blind, randomized, multicenter controlled trial. Circulation. 2000. 102(4):426–431.

10. Mohler ER 3rd, Hiatt WR, Olin JW, Wade M, Jeffs R, Hirsch AT. Treatment of intermittent claudication with beraprost sodium, an orally active prostaglandin I2 analogue: a double-blinded, randomized, controlled trial. J Am Coll Cardiol. 2003. 41(10):1679–1686.

11. Toyota T, Oikawa S. Beraprost Sodium Study Group. Effects of beraprost sodium (Dorner) in patients with diabetes mellitus complicated by chronic arterial obstruction. Angiology. 2002. 53(1):7–13.

12. Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962. 27(6):645–658.
13. Taylor LM, Porter JM. Proposed design for a double blinded trial to evaluate medications for treatment of intermittent claudication. J Vasc Surg. 1992. 15(5):882–884.

14. Newman AB, Siscovick DS, Manolio TA, et al. Cardiovascular Heart Study (CHS) Collaborative Research Group. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993. 88(3):837–845.

15. Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation. 1985. 71(3):516–522.

16. Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993. 270(4):465–469.

17. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975. 1(3):277–299.

18. Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003. 19(5):306–314.

19. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006. 47(5):921–929.

20. Dawson DL, Cutler BS, Hiatt WR, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000. 109(7):523–530.

21. Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998. 98(7):678–686.

22. Beebe HG, Dawson DL, Cutler BS, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med. 1999. 159(17):2041–2050.

23. Belch JJ, Bell PR, Creissen D, et al. Randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of AS-013, a prostaglandin E1 prodrug, in patients with intermittent claudication. Circulation. 1997. 95(9):2298–2302.

24. Müller-Bühl U, Diehm C, Krais T, Zimmermann R, Morl H, Eckstein HH. Clinical effects of intravenous iloprost in patients with intermittent claudication. Eur J Clin Pharmacol. 1987. 33(2):127–131.

25. Daousi C, Benbow SJ, Woodward A, MacFarlane IA. The natural history of chronic painful peripheral neuropathy in a community diabetes population. Diabet Med. 2006. 23(9):1021–1024.

26. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006. 29(7):1518–1522.

27. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. KORA Study Group. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008. 31(3):464–469.

28. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009. 10(2):393–400.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download