INTRODUCTION
Solitary fibrous tumor (SFT) is a rare neoplasm that was initially identified as a pleura-based mass by Klemperer and Rabin in 1931. Since its initial description, SFT has been described in various body cavities, soft tissue, and visceral organs, but is rarely reported in breast tissue; to date, only 27 cases of breast SFT have been reported in the literature (summarized in
Supplementary Table 1).
SFT may be found incidentally or due to nonspecific symptoms, such as a palpable mass or mass effects, depending on the size and location. Paraneoplastic syndromes have also been reported. The prototypical histology of SFT is a well-defined mass composed of spindle cells arranged in a “patternless” pattern with alternating hypercellular and hypocellular areas, a characteristic hemangiopericytic vascular pattern, the so-called “staghorn” appearance, and ropy collagen. Secondary changes, such as myxoid change, cystic degeneration, hemorrhage, necrosis, and calcifications, are more frequently found in larger SFTs and are attributed to heterogeneous radiologic findings of the intralesional geographic pattern, in contrast to the homogeneous pattern in smaller SFTs.
Most SFTs have an indolent clinical course; however, some SFTs may recur, metastasize, and cause death. Many pathologic parameters are associated with poor prognosis including: large tumor size, positive margins, increased nuclear pleomorphism, necrosis, hemorrhage, vascular invasion, high mitotic count (≥ 4 mitoses/10 high-power fields [HPFs]), and increased Ki67 labeling index. Because aggressive prognoses have been reported in SFT, even with bland histologic features, SFT is best categorized as “borderline tumor of uncertain malignant potential, rarely metastasizing.”
Herein, we report a rare case of SFT in the breast with areas of malignant SFT, and we review other reported cases of breast SFTs described in the literature. This case showed areas of malignant histology, including increased cellularity, nuclear pleomorphism, areas of necrosis and hemorrhage, decreased vascularity, high mitotic count (9 mitoses/10 HPFs), and increased Ki67 labeling index (25%). The main question addressed was the differential diagnosis between malignant SFT and dedifferentiated SFT. In these areas, cluster of differentiation (CD) 34, B-cell lymphoma 2 (Bcl-2), and signal transducer and activator of transcription 6 (STAT6) immunostains showed diffuse and strong immunoreactivities as seen in the classic areas of SFT. Based on these immunoreactivities and gradual transition from the classic area, malignant SFT was diagnosed over dedifferentiated SFT. To our knowledge, only one other case of malignant SFT in the breast has been reported in the English literature.
CASE REPORT
A 75-year-old Caucasian woman presented to our breast surgery clinic with a 3-month history of a palpable left upper outer quadrant breast mass. She denied any other symptoms, such as pain, nipple discharge, or skin changes. She had undergone 3 previous excisional biopsies of the right breast over 40 years. All biopsy results showed that the tumors were benign, with no evidence of spindle cell neoplasm, atypical hyperplasia, or malignancy. She had no family history of breast or ovarian cancer, or other malignancy. She had menarche at age 13 and had undergone hormone replacement therapy for 12–15 years after total hysterectomy and bilateral salpingo-oophorectomy at age 48 years for leiomyomata of the uterus.
On physical examination, there was a soft mass in the deep left upper outer quadrant measuring 4.5 × 4.0 cm. The mass was at the 1 o'clock position, 8 cm from the nipple. There was no skin change, nipple discharge, or axillary lymphadenopathy noted. Diagnostic breast imaging revealed an oval circumscribed highly vascularized hypoechoic mass in the left breast at the 1 o'clock position, 11 cm from the nipple, measuring 4.5 × 4.0 × 2.8 cm (
Figure 1A and B). As the mass was highly vascularized, needle biopsy was not attempted and the patient was referred for surgical resection.
Figure 1
Radiologic findings (A) Ultrasound showing a highly vascular, oval, circumscribed, and hypoechoic mass in the left breast at posterior depth causing mass effect on the pectoralis muscle posteriorly. (B) Mammogram shows an oval, circumscribed, and obscured mass in the left breast at posterior depth abutting the pectoralis muscle. This mass contains a single benign-appearing coarse calcification.
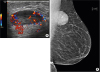
Upon surgical resection, the mass was found to be a soft, purple, and well-circumscribed lesion. Blunt finger dissection was used to separate the encapsulated mass from the surrounding breast tissue, although it appeared focally fixed to the underlying pectoralis major fascia. The underlying fascia was removed with the specimen to avoid violating the thin capsule.
Grossly, the specimen was a solid mass measuring 4.4 × 3.5 × 2.8 cm. The outer and cut surfaces were homogeneously pink and smooth with focal discoloration; no necrosis or hemorrhage was evident. Microscopically, the mass was well circumscribed. However, focal infiltration into the surrounding skeletal muscle and adipose tissue was identified (
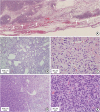
Figure 2A). The mass was composed of plump-to-ovoid spindle cells intermingled with fibrillary collagen fibers and alternating hypercellular and hypocellular areas (
Figure 2B and C). Dilated small-to-large vasculature was dispersed throughout these regions, some of which exhibited a prominent staghorn-like branching pattern (
Figure 2B). In focal areas, enlarged and pleomorphic tumor cells were arranged in compact sheet-like patterns with diminished branching vasculature. A gradual transition between these and classic tumor areas was observed (
Figure 2D). In the hypercellular and pleomorphic areas, the tumor nuclei were rounder with high mitotic counts, up to 9/10 HPFs (
Figure 2E). In the periphery, hyalinized fibrotic nodules with microcalcifications were identified. However, amianthoid fibers or entrapped mammary ducts/lobules were absent.
Figure 2
Microscopic findings (A) Well demarcated tumor border; however, it is focally permeative into the surrounding adipose tissue and skeletal muscles (arrows). (B) Tumor cells are arranged haphazardly with alternating cellularity (hypocellular on left and hypercellular on right) and prominent vasculature. (C) Hypocellular area is composed of bland looking, plump spindle or oval tumor cells. (D) Hypercellular area with compact sheet-like arrangement (left side) is transited from classic tumor areas (right side). (E) Hypercellular area is composed of pleomorphic tumor cells with increased mitotic figures (hematoxylin and eosin stain; (A) ×50, (B) ×100, (C) ×400, (D) ×100, and (E) ×400).
We performed immunohistochemical staining for CD34, STAT6, Bcl-2, cytokeratin, S100, p63, and Ki67. The antibodies used are listed in
Table 1. Tumor cells were positive for CD34, STAT6, and Bcl-2 in the classic and anaplastic areas and negative for cytokeratin, S100, and p63 in both areas (
Figure 3). The Ki67 labelling index in anaplastic areas was 25%, whereas it was < 5% in classic areas. In the hypercellular and pleomorphic areas, CD34, Bcl-2, and STAT6 showed the same intensity of immunoreactivity (
Figure 4). Based on the morphological and immunohistochemical findings, the lesion was diagnosed as SFT with anaplastic areas (malignant SFT) of the breast.
Table 1
Details of immunohistochemical markers
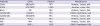
|
Antibody |
Clone |
Dilution |
Manufacturer |
|
CD34 |
QBEnd/10 |
RTU |
Ventana, Tucson, USA |
|
STAT6 |
GTX113273 |
1:500 |
GeneTex, Irvine, USA |
|
Bcl-2 |
124 |
RTU |
Ventana, Tucson, USA |
|
Cytokeratin |
AE1/AE3 |
RTU |
DAKO, Carpinteria, USA |
|
Cytokeratin |
34betaE12 |
RTU |
Ventana, Tucson, USA |
|
S100 |
4C4.9 |
RTU |
Ventana, Tucson, USA |
|
P63 |
4A4 |
RTU |
Ventana, Tucson, USA |
|
Ki67 |
MIB-1 |
1:100 |
DAKO, Carpinteria, USA |
Figure 3
Tumor cells positive for cluster of differentiation 34 (A), signal transducer and activator of transcription 6 (B), B-cell lymphoma 2 (C) negative for cytokeratin (D), S100 (E), and p63 (F) (×200).
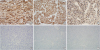
Figure 4
Immunohistochemical findings in hypercellular and pleomorphic areas cluster of differentiation 34 (A), B-cell lymphoma 2 (B), and signal transducer and activator of transcription 6 (C). Immunoreactivity shows the same staining pattern and intensity as in classic areas (×200).

After surgical enucleation, the case was presented to the Institutional Sarcoma Tumor Board. The patient underwent staging work-up with chest imaging and there was no evidence of metastatic disease. She returned to the operating room for wide margin re-excision and had no residual disease. She is undergoing close follow-up with no further treatment and had no evidence of recurrence at 5 months.
DISCUSSION
SFT of the breast is exceedingly rare. To our knowledge, only 27 cases have been described as single cases or small case series in the English literature (summarized in
Supplementary Table 1). Overall, patients with these lesions present with a palpable mass of varying size, ranging from 0.6 cm to 10 cm with a mean of 3.5 cm. The side of breast involvement is roughly similar, with 9 cases in the left and 11 cases in the right breast (7 cases, side not mentioned). The age of patients is in the range 38 to 88 years with a mean age of 60 years. SFTs of the breast are more commonly seen in female (19 cases) than male (8 cases) patients, in contrast to a similar rate of overall SFT cases between males and females. This discordant prevalence may be attributed to the judgment as to the epicenter of SFT. Most reported cases exhibit CD34, STAT5, Bcl-2, and CD99 positivity.
As with SFTs from other anatomic locations, mammary SFTs are characterized by a well-defined mass composed of bland oval to spindle cells arranged in a haphazard “patternless” pattern, alternating with hypercellular and hypocellular areas. In the hypocellular areas, there is a characteristic hemangiopericytic vascular pattern, the so-called “staghorn” vessels, in a rich, collagenous stroma with ropy collagen. However, nonclassical SFTs have also been documented. In these cases, oval to spindle cells may be arranged in various patterns, such as sheet-like, storiform, fascicular, and even a herringbone pattern. The vessels may be simply dilated without branching, and their diameters can vary from capillary to large vessels. The stroma may exhibit secondary changes, such as myxoid change, cystic degeneration, hemorrhage, necrosis, and calcifications, particularly in larger SFTs. These nonclassical microscopic findings can often confound the diagnosis.
When mammary lesions present with spindle cell formation and limited characteristics of SFT, they need to be differentiated from other spindle cell lesions of the breast. For the differential diagnosis, the basic and most important considerations are the histologic findings, supported by ancillary studies and correlation with the clinical and radiologic findings. The common spindle cell lesions of the breast can be grouped according to cytologic features. For low-grade lesions, common considerations are the following: exuberant scar, schwannoma, neurofibroma, desmoid-type fibromatosis, fibromatosis-like variant of metaplastic carcinoma, and benign or borderline phyllodes tumor. Careful histologic examination with proper selection of immunohistochemical stains can easily differentiate SFT from these spindle cell lesions. These include the following: diffuse immunoreaction for S100 in schwannoma; combined immunoreaction for S100, CD34, and epithelial membrane antigen (EMA) in Schwann cells, fibroblasts, and perineural cells in neurofibroma; nuclear immunoreaction for beta-catenin and mutation of CTNNB1 and APC genes in desmoid-type fibromatosis; and immunoreaction for cytokeratin and p63 in fibromatosis-like variant of metaplastic carcinoma.
Generally, SFT shows diffuse and strong expression of CD34, Bcl-2, and CD99. However, a similar staining profile has been reported in myofibroblastoma and spindle cell lipoma in the breast [
1]. Furthermore, these entities may exhibit overlapping gross and microscopic findings. Based on these similarities, Magro et al. [
2] adopted the comprehensive term “benign spindle stromal tumors of the breast,” encompassing SFT, myofibroblastoma, and spindle cell lipoma. Furthermore, the authors suggested a unified histogenetic concept, which considered vimentin- and CD34-positive uncommitted stromal cells of normal mammary stroma as the common precursor cells, with plasticity.
In addition to the overlapping microscopic findings, spindle cell lipoma and mammary-type myofibroblastoma share a genetic alteration on chromosome 13. Partial monosomy of chromosome 13q results in the monoallelic deletion of retinoblastoma (Rb)/13q14 and forkhead box protein O1/13q14, resulting in subsequent loss of Rb protein expression [
3]. Hence, mammary myofibroblastoma and spindle cell lipoma have been considered to be linked disease entities, the so-called 13q/Rb family of tumors. These genetic alterations are detected with fluorescent in-situ hybridization analysis showing the monoallelic deletion of 13q14 loci, or with immunohistochemistry for Rb showing loss of nuclear immunoreactivity. Interestingly, a few studies on genetic alterations in SFT have reported similar genetic alterations, such as loss of chromosome 13q [
4]. However, some recent studies report that deletions in 13q, even the most frequently deleted region, are identified in only 4% of SFTs [
5]. Moreover, these studies have found that SFTs tested by whole genome high-resolution array-based comparative genomic hybridization are “genetically simple,” which means that SFTs lack high genetic instability. In support of this, Fritchie et al. [
6] reported the absence of genetic alteration of 13q in a study on 40 cases of SFTs, compared with alterations in a small number of cases of cellular angiofibroma, spindle cell lipoma, and mammary-type myofibroblastoma. These discrepancies might be due to the different criteria of assignment into each disease entity with overlapping microscopic findings, such as a well-defined mass composed of uniform spindle cells with CD34 immunoreactivity and collagenous deposits. In fact, many studies assessing genetic alterations in these tumors do not describe the diagnostic criteria of each disease. Therefore, although the terms SFT, myofibroblastoma, and spindle cell lipoma are often used interchangeably in the breast, the elucidation of their relationship requires further study.
Recently, STAT6 has been identified as a surrogate immunohistochemical marker to distinguish SFT from other spindle cell tumor-like and tumor lesions [
7]. This chimeric protein is formed via somatic fusion of a tumor suppressor gene,
STAT6, with an adjoining and partially overlapping transcription factor gene,
NAB2, induced by the paracentric inversion at chromosomal region 12q13, presumably a tumor-initiating event in SFTs [
8].
STAT6 nuclear staining, which is typically diffuse and intense, is indicative of SFT [
9]. However, nuclear STAT6 has also been reported in cases of meningeal hemangiopericytoma and pulmonary adenofibroma, which are considered within the spectrum of SFT, and in some non-SFT-related lesions, such as nodular fasciitis (2%), myxoid/round cell liposarcoma (11%), dedifferentiated liposarcoma (7%), undifferentiated pleomorphic sarcoma (2%), low-grade fibromyxoid sarcoma (29%), and ovarian fibroma, albeit with a limited number of reported cases [
9101112]. However, to our knowledge, diffuse and intense nuclear immunoreaction is not identified in mammary or non-mammary low-grade spindle cell lesions, such as classic-type myofibroblastoma, spindle cell lipoma, benign fibroblastic spindle cell tumor, pseudoangiomatous stromal hyperplasia, reactive spindle cell nodule, desmoid-type fibromatosis, and spindle cell metaplastic carcinoma [
7].
In our case, besides typical areas of SFT, areas of the tumor showed atypical epithelioid cells arranged in hypercellular sheets with diminished branching vasculature. These areas showed nuclear pleomorphism and increased mitotic count (up to 9/10 HPFs) associated with high Ki67 level (25%). The main question in this case was whether this component represented a malignant SFT versus dedifferentiated SFT. Our case was favored to be a malignant form of SFT based on malignant histologic features with cytologic atypia, increased mitoses (≥ 4/10 HPFs), infiltrative margins, and preservation of CD34, Bcl-2, and STAT6 immunoreactivity. Malignant SFT has previously been described only once in the breast by Yang et al. [
13].
There are notable distinctions between malignant and dedifferentiated SFTs. Both have classic and atypical components; however, in malignant SFTs, 2 components show gradual transition with intimate arrangement of both components, as seen in our case. Mosquera and Fletcher [
14] defined dedifferentiation of SFT as an abrupt transition of bland SFT into high-grade sarcoma, regardless of pattern; they reported that high-grade tumor cells in dedifferentiated areas are arranged in hypercellular sheets composed of either only epithelioid cells or a mixture of epithelioid, round, and spindle cells. Those authors found that cystic degeneration and necrosis were more common in high-grade areas than in low-grade areas; therefore, selection of regions with cystic or necrotic areas during gross examination may be helpful for the detection of dedifferentiated component, even in tumors with well-defined borders. Dedifferentiated SFT is a very rare finding, with less than 1% (8 cases) of 948 cases of SFT described in the 21-year consultation files of Mosquera and Fletcher [
14].
Before making a diagnosis of dedifferentiated SFT, pathologists need to differentiate other high-grade lesions, such as malignant phyllodes tumor, high-grade spindle cell metaplastic carcinoma, and sarcoma. Diagnosing malignant phyllodes tumor may be straightforward when the biphasic components of stromal and epithelial elements and leaf-like fronds are identified. However, some malignant phyllodes tumors can present as a high-grade spindle cell nodule with extensive stromal overgrowth. When there are diagnostic difficulties even after extensive sampling, increased immunoreactivity for CD117 may aid with the diagnosis of malignant phyllodes tumor.
Spindle cell metaplastic carcinoma is composed of obviously atypical spindle cells arranged in various patterns; it typically has an infiltrative border, but a pushing border may also be present. Accompanying ductal carcinoma in situ or carcinoma component showing ductal or squamous differentiation are helpful features for microscopic diagnosis. However, if these features are not evident, the immunohistochemical findings are crucial, and include positive immunoreaction for cytokeratin or EMA and negative immunoreaction for CD34, STAT6, and Bcl-2.
Interestingly, several cases of SFT harboring metastatic carcinoma have been described as a very rare recipient of tumor-to-tumor metastasis, the most common metastatic donor being breast carcinoma [
15]. This phenomenon may be explained by the rich vascularity in SFT, like in renal cell carcinoma, which is the most common recipient of tumor-to-tumor metastasis. In this situation, the main differential diagnosis should be spindle metaplastic carcinoma.
Furthermore, the dedifferentiated high-grade component in SFT may exhibit no immunoreactivity or reduced immunoreactivity for CD34, STAT6, and CD99 compared with classic or malignant SFT; hence, immunohistochemical assessment in cases with suspected SFT may be more informative in low-grade lesions [
14]. Thus, immunohistochemical interpretation for high-grade spindle cell lesion with unknown origin needs to be carefully performed with comprehensive correlation of overall histologic findings and immunohistochemical assessment using the proper panels, including CD34, STAT6, and Bcl-2.
Generally, the primary treatment for localized SFT is complete en bloc surgical resection. For locally advanced, recurrent, or metastatic SFTs, both systemic and targeted therapies have been investigated; however, there is no standardized therapy for these tumors. Although there are no studies on therapeutic modality and post-treatment monitoring focusing on mammary SFT, complete surgical resection and long-term monitoring of SFT to detect late recurrence should be considered.
In conclusion, we report a rare case of mammary SFT with anaplastic malignant areas. The microscopic and immunohistochemical findings were similar to those of SFTs identified in other anatomical sites. The specific diagnosis of SFT is needed for proper treatment and monitoring, rather than assigning ambiguous encompassing terminology. With comprehensive pathologic diagnosis with proper sampling, full awareness of the similarities and differences between SFT and mimicking lesions, and proper ancillary testing including STAT6 assessment, an accurate diagnosis of SFT can be established.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download