Abstract
Purpose
The recent trend in breast cancer treatment is to minimize axillary dissection. However, no pattern of axillary metastasis has been precisely established. The purpose of this study was to evaluate the metastatic lymphatic pattern using near-infrared fluorescence imaging with indocyanine green (ICG) in breast cancer with cytologically proven axillary metastasis.
Methods
This was a prospective single-center study. We evaluated 147 patients with breast cancer involving cytologically proven axillary metastasis, and compared physiological and nonphysiological lymphatic metastasis.
Results
We performed lymphatic mapping for 64 patients who exhibited level II lymphatic flow on near-infrared fluorescence imaging with ICG, and found that all had axillary metastasis: 51 patients who did not receive neoadjuvant chemotherapy (NAC) and 13 patients post-NAC. Of patients who did not receive NAC, 32 had physiological lymphatic metastasis and 19 had nonphysiological lymphatic metastasis. The risk factors for nonphysiological lymphatic metastasis were age ≥55 years, high Ki-67 index (>20%), and perinodal extension in both univariate and multivariate analysis (p<0.05).
Axillary staging remains a primary prognostic discriminant in breast cancer and is important for tailoring treatment [1]. Recent studies have shown that sentinel lymph node (SLN) biopsy without axillary lymph node dissection (ALND) offers excellent regional control, and this regimen is likely to be a reasonable option for management [23].
SLN biopsy indicates that the first nodes in the lymphatic chain are at risk for metastasis, and post-SLN metastasis reflects the involvement of subsequent nodes. However, the pattern of axillary metastasis has not yet been defined. While SLN metastasis is well known, few studies have investigated post-SLN metastasis. The transition to non-SLNs through nonphysiological lymphatic flow is of interest in breast cancer.
Indocyanine green (ICG) is considered an accurate tool for studying patterns of metastasis [45]. Several studies have shown that ICG is a safe and practical compared blue dye and isotope [6789]. Furthermore, ICG has been shown to identify SLN at rates greater than 90%. Injection of ICG allows real-time transcutaneous lymphography with direct visualization of the lymphatic pathways, which can be traced to the axilla [791011]. Near-infrared (NIR) fluorescence imaging provides visualization of subcutaneous lymphatic flow and allows surgeons to directly observe the axillary nodes [8]. ICG is more effective at traveling to the lymph nodes after the surveillance lymph node, compared with the conventional blue or radioactive colloid pigments [5].
The purposes of this study were to evaluate the lymphatic pattern by using ICG in breast cancer with cytologically proven axillary metastasis, and to investigate risk factors for nonphysiological lymphatic metastasis.
In this prospective single-arm study, we included 147 patients diagnosed with invasive breast cancer with cytologically proven axillary lymph node metastasis, who then underwent curative surgery at Samsung Medical Center between May 2016 and December 2017 and three breast surgeons participated. Sixty-eight patients had received neoadjuvant chemotherapy (NAC) and 79 patients did not receive NAC. Of the 147 total patients, 31 experienced ICG failure because of whole or inflammatory breast cancer, and 28 achieved axillary pathologic complete response after NAC. Among the 88 remaining patients, 64 displayed axillary level II lymphatic flow on NIR fluorescence imaging with ICG and 24 patients without axillary level II lymphatic flow on ICG were excluded. We evaluated these patients and performed lymphatic mapping with ICG. For accurate analysis, we divided the patients into without NAC and post-NAC.
For the ICG staining, ICG was diluted 100 times, and then 5 cc of the diluted ICG was injected intradermally and subcutaneously into the breast using a 24-gauge needle. Each patient then underwent ALND, which was performed using a fluorescence camera. First, we removed the fluorescent lymph node at axillary levels I and II. We then removed the remaining axillary lymph nodes at levels I and II. We obtained the results for the axillary lymph node by classification (Figures 1 and 2).
In this study, the non-SLN was defined as the lymph node that was not stained along with the ICG node but as an existing lymph node at axillary level I. Physiologic lymphatic metastasis was defined as metastasis that was SLN-positive at axillary level I, non-SLN-negative and axillary level II lymph-node–negative or positive. Nonphysiological lymphatic metastasis was defined as the other physiology pattern of lymphatic metastasis, with nonfluorescent lymph node metastasis by ICG (Figures 3 and 4).
We used the chi-square test and Spearman correlation coefficient to compare discrete variables. Differences were assumed to be statistically significant when the p-value was less than 0.05. We used SPSS version 23 (IBM Inc., Armonk, USA) for the chi-square tests and for Spearman correlation coefficient. This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea (IRB file no. 2015-01-046). All patients provided written informed consent.
We performed lymphatic mapping for 64 patients with level II lymphatic flow using ICG. Among 64 patients, the without NAC group comprised 51 patients and the post-NAC group comprised 13 patients. Without NAC group, there were 32 patients with SLN metastasis and without non-SLN metastasis, and all of them had physiological lymphatic metastasis. Of these patients, two (6.3%) had lymph node metastasis in level II and 30 patients (93.1%) did not have lymph node metastasis in level II. The number of patients with non-SLN metastasis was 19, of which 10 (52.6%) had lymph node metastasis in level II and nine (47.3%) patients had no lymph node metastasis in level II (Figure 3). In the post-NAC group, there were five patients with SLN metastasis and without non-SLN metastasis, and all of these had physiologic lymphatic metastasis. Among these patients, one (20.0%) had lymph node metastasis in level II and four patients (80.0%) did not have lymph node metastasis in level II. The number of patients with non-SLN metastasis was eight, of which seven (87.5%) had lymph node metastasis in level II and one (12.5%) patients had no lymph node metastasis in level II (Figure 4).
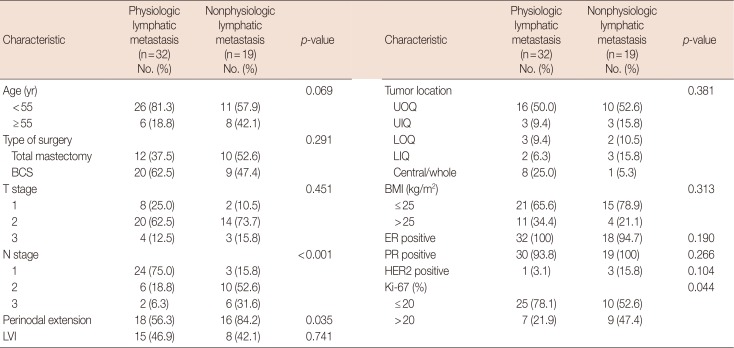
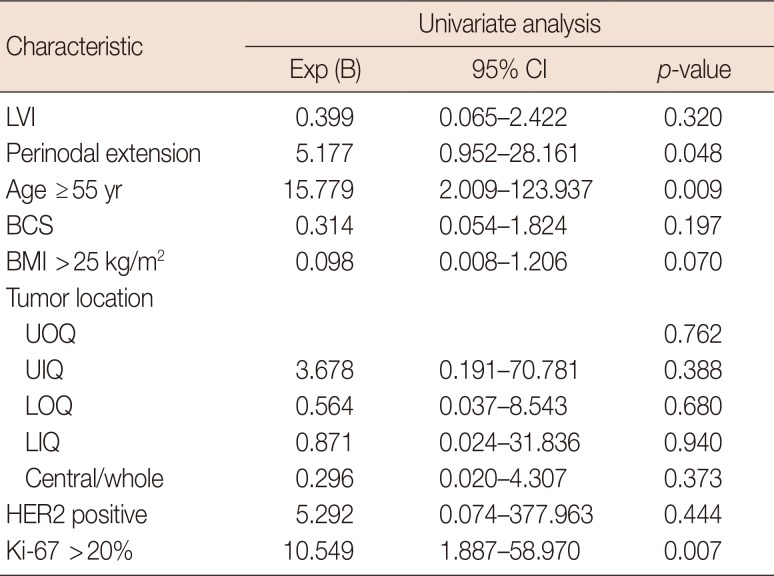
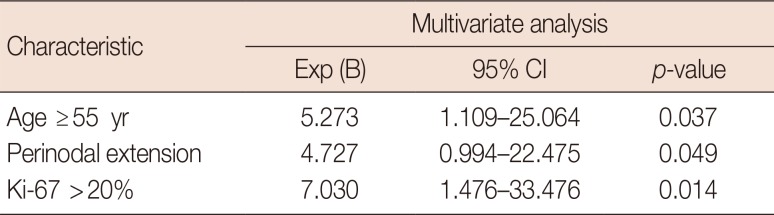
Of 51 patients who did not receive NAC, 32 had axillary metastasis with physiologic lymphatic metastasis and 19 patients had nonphysiological lymphatic metastasis. We compared the clinicopathological characteristics between these groups (Table 1). The risk factors for nonphysiologic lymphatic metastasis were high Ki-67 index (>20%), perinodal extension (PNE), and age ≥55 years in both univariate and multivariate analyses (p<0.05) (Tables 2 and 3).
In this prospective study, we performed lymphatic flow imaging using ICG of cytologically proven axillary metastasis. Imaging was used for intraoperative navigation to identify SLNs and non-SLNs. The results of previous studies regarding SLN detection by NIR fluorescence using ICG for breast cancer showed that the identification rate of SLNs was 92%–100% [4912131415]. This indicates that the discrimination of SLNs using ICG is very reliable.
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial investigated women with T1 or T2 invasive primary breast cancer and 1 or 2 SLNs containing metastases. In this trial, the 10-year overall survival for patients treated with SLN dissection alone was noninferior to that for those treated with ALND [3]. In the After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trial, SLN biopsy without ALND offered excellent regional control, indicating that this regimen may be a reasonable option for management [2]. Although our study involved patients who were axillary lymph node-positive before surgery, 32 patients had positive SLNs and negative non-SLNs, including two patients (6.3%) with level II lymph node metastasis.
A recent study determined the percentage of patients with invasive breast cancer who had N2 or N3 stage disease, and developed a nomogram for the prediction of N2 or N3 stage disease in these patients. The nomogram revealed that lymphovascular invasion, T2 stage, metastatic lymph node ratio, and PNE were independent predictors of N2 or N3 stage disease [16]. PNE, found in frozen sections from breast cancer, is a predictor [17]. Our study showed that 29 of 35 patients (82.9%) with N2 or N3 stage disease had PNE, and 31 of 35 patients (88.6%) with N2 or N3 stage disease had stage T2 or greater disease.
Selective ALND involves only the fluorescent lymph nodes at level I and II visualized using ICG. Selective dissection may lead to less arm morbidity and lymphedema, compared with routine ALND. A study showed that selective ALND after reverse mapping prevented breast-cancer–related lymphedema [18]. However, patients aged ≥55 years with high Ki-67 indices (>20%) and perinodal status, such as those in our study, might not be good candidates for selective axillary dissection, as these patients may have nonphysiological lymphatic metastasis.
Skip metastasis of axillary lymph nodes is an important phenomenon. The mechanism of skip metastasis of axillary lymph node is unclear. In some article, skip metastasis has been defined as level I absence but level II and/or III involvement [19]. However skip metastasis was not observed in our study. Chemotherapy can induce axillary lymph node fibrosis and lymphatic flow block. Nonphysiologic lymphatic metastasis may be affected by these factors. In our study, eight (61.5%) and 19 patients (37.3%) were found in the post-NAC and without NAC groups with nonphysiological lymphatic patterns, respectively. There was no statistical difference (p=0.114) but patients in the post-NAC group showed a greater tendency of non-physiological lymphatic metastasis.
This study had several limitations. First, it was limited to a single comprehensive cancer institution. Second, our study was a prospective design study of lymphatic passage in patients with cytologically proven axillary metastasis, and we analyzed only those patients in whom SLN metastasis was confirmed and all ICG levels were elevated to level II. Therefore, it is difficult to mention the identification rate of SLNs and false negative rate of SLNs using ICG in this study. Third, this study did not enroll a large population, limiting the statistical power. However, this study is meaningful even if the number of patients was relatively small, because it had a prospective design with clinically suspected node-positive patients confirmed by cytology. In addition, there has been little research conducted regarding post-SLN metastasis. Furthermore, we obtained the results of axillary lymph nodes in axillary level I (SLNs and non-SLNs) and level II lymph nodes by classification.
In conclusion, lymphatic metastatic flow in cytologically proven axillary metastasis can be either physiological or nonphysiological lymphatic metastasis. Axillary metastases in nonphysiological lymphatic flow are more frequently level II lymph node metastases. In without NAC group, patients with cytologically proven axillary metastasis aged ≥55 years, with high Ki-67 indices (>20%), and with PNE may have nonphysiological lymphatic metastasis. Patients with these risk factors might not be good candidates for selective axillary dissection.
References
1. Dewis R, Gribbin J. National Institute for Health and Clinical Excellence: guidance. In : Dewis R, Gribbin J, editors. Breast Cancer: Diagnosis and Treatment: An Assessment of Need. Cardiff: National Collaborating Centre for Cancer;2009. p. 13–35.
2. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde C, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014; 15:1303–1310. PMID: 25439688.

3. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017; 318:918–926. PMID: 28898379.
4. Guo J, Yang H, Wang S, Cao Y, Liu M, Xie F, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol. 2017; 15:196. PMID: 29096643.

5. Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011; 104:323–332. PMID: 21495033.

6. Jung SY, Kim SK, Kim SW, Kwon Y, Lee ES, Kang HS, et al. Comparison of sentinel lymph node biopsy guided by the multimodal method of indocyanine green fluorescence, radioisotope, and blue dye versus the radioisotope method in breast cancer: a randomized controlled trial. Ann Surg Oncol. 2014; 21:1254–1259. PMID: 24356798.

7. Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010; 121:373–378. PMID: 20140704.

8. Sugie T, Kassim KA, Takeuchi M, Hashimoto T, Yamagami K, Masai Y, et al. A novel method for sentinel lymph node biopsy by indocyanine green fluorescence technique in breast cancer. Cancers (Basel). 2010; 2:713–720. PMID: 24281090.

9. Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol. 2012; 38:651–656. PMID: 22704050.

10. Alford R, Simpson HM, Duberman J, Hill GC, Ogawa M, Regino C, et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging. 2009; 8:341–354. PMID: 20003892.

11. Gilmore DM, Khullar OV, Gioux S, Stockdale A, Frangioni JV, Colson YL, et al. Effective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanoma. Ann Surg Oncol. 2013; 20:2357–2363. PMID: 23440551.

12. Hokimoto N, Sugimoto T, Namikawa T, Funakoshi T, Oki T, Ogawa M, et al. A novel color fluorescence navigation system for intraoperative transcutaneous lymphatic mapping and resection of sentinel lymph nodes in breast cancer: comparison with the combination of gamma probe scanning and visible dye methods. Oncology. 2018; 94:99–106. PMID: 29131021.

13. Zhang X, Li Y, Zhou Y, Mao F, Lin Y, Guan J, et al. Diagnostic performance of indocyanine green-guided sentinel lymph node biopsy in breast cancer: a meta-analysis. PLoS One. 2016; 11:e0155597. PMID: 27280407.

14. Chi C, Ye J, Ding H, He D, Huang W, Zhang GJ, et al. Use of indocyanine green for detecting the sentinel lymph node in breast cancer patients: from preclinical evaluation to clinical validation. PLoS One. 2013; 8:e83927. PMID: 24358319.

15. Pitsinis V, Provenzano E, Kaklamanis L, Wishart GC, Benson JR. Indocyanine green fluorescence mapping for sentinel lymph node biopsy in early breast cancer. Surg Oncol. 2015; 24:375–379. PMID: 26555151.

16. Kim I, Ryu JM, Kim JM, Choi HJ, Lee SK, Yu JH, et al. Development of a nomogram to predict N2 or N3 stage in T1-2 invasive breast cancer patients with no palpable lymphadenopathy. J Breast Cancer. 2017; 20:270–278. PMID: 28970853.

17. van der Loo EM, Sastrowijoto SH, Bril H, van Krimpen C, de Graaf PW, Eulderink F. Less operations required due to perioperative frozen section examination of sentinel nodes in 275 breast cancer patients. Ned Tijdschr Geneeskd. 2001; 145:1986–1991. PMID: 11680071.
18. Gennaro M, Maccauro M, Sigari C, Casalini P, Bedodi L, Conti AR, et al. Selective axillary dissection after axillary reverse mapping to prevent breast-cancer-related lymphoedema. Eur J Surg Oncol. 2013; 39:1341–1345. PMID: 24113621.

19. Wang H, Mao XY, Zhao TT, Zheng XY, Jin F, Li JG. Study on the skip metastasis of axillary lymph nodes in breast cancer and their relation with Gli1 expression. Tumour Biol. 2012; 33:1943–1950. PMID: 22797820.

Figure 1
Axillary lymph node dissection with lymphatic mapping on indocyanine green. (A) Axillary lymph node dissection seen in surgical field. (B) Axillary lymph node dissection seen in near-infrared fluorescence.
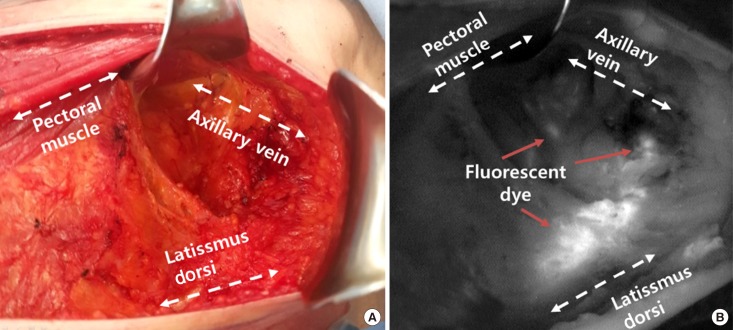
Figure 2
Classification of axillary lymph node dissection by indocyanine green. (A) Level I sentinel lymph node in near-infrared fluorescence. (B) Level I non-sentinel lymph node in near-infrared fluorescence. (C) Level II lymph node in near-infrared fluorescence.
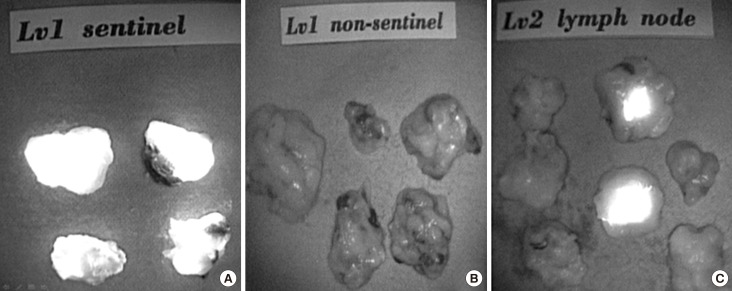
Figure 3
Algorithm of axillary metastasis between physiologic lymphatic metastasis and nonphysiologic lymphatic metastasis without neoadjuvant chemotherapy.
LN=lymph node; SLN=sentinel lymph node.
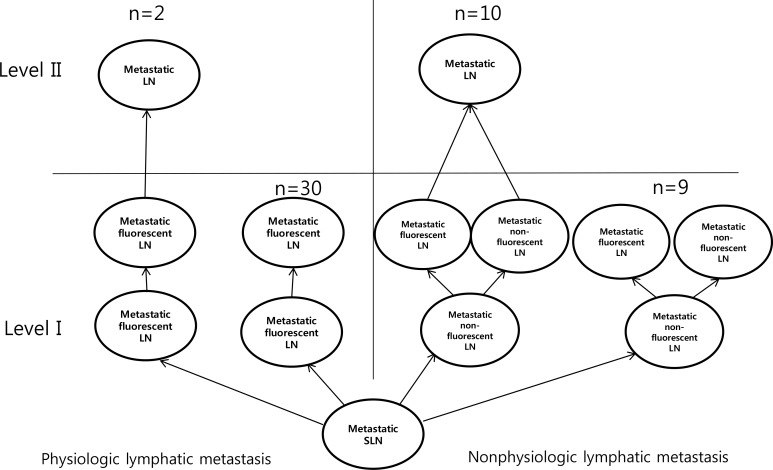
Figure 4
Algorithm of axillary metastasis between physiologic lymphatic metastasis and nonphysiologic lymphatic metastasis with neoadjuvant chemotherapy.
LN=lymph node; SLN=sentinel lymph node.
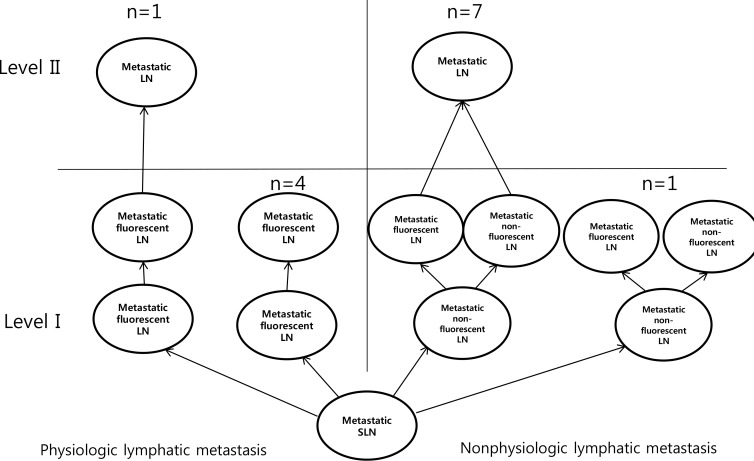
Table 1
Comparison of clinicopathological characteristics between patients with physiologic versus nonphysiologic lymphatic metastasis without neoadjuvant chemotherapy




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download