Abstract
Purpose
There is still a clinical need to easily evaluate the metastatic status of lymph nodes during breast cancer surgery. We hypothesized that ex vivo shear-wave elastography (SWE) would predict precisely the presence of metastasis in the excised lymph nodes.
Methods
A total of 63 patients who underwent breast cancer surgery were prospectively enrolled in this study from May 2014 to April 2015. The excised axillary lymph nodes were examined using ex vivo SWE. Metastatic status was confirmed based on the final histopathological diagnosis of the permanent section. Lymph node characteristics and elasticity values measured by ex vivo SWE were assessed for possible association with nodal metastasis.
Results
A total of 274 lymph nodes, harvested from 63 patients, were examined using ex vivo SWE. The data obtained from 228 of these nodes from 55 patients were included in the analysis. Results showed that 187 lymph nodes (82.0%) were nonmetastatic and 41 lymph nodes (18.0%) were metastatic. There was significant difference between metastatic and nonmetastatic nodes with respect to the mean (45.4 kPa and 17.7 kPa, p<0.001) and maximum (55.3 kPa and 23.2 kPa, p<0.001) stiffness. The elasticity ratio was higher in the metastatic nodes (4.36 and 1.57, p<0.001). Metastatic nodes were significantly larger than nonmetastatic nodes (mean size, 10.5 mm and 7.5 mm, p<0.001). The size of metastatic nodes and nodal stiffness were correlated (correlation coefficient of mean stiffness, r=0.553). The area under curve of mean stiffness, maximum stiffness, and elasticity ratio were 0.794, 0.802, and 0.831, respectively.
Elastography is a promising method in the assessment of differences in tissue stiffness and in distinguishing benign from malignant lesions [123]. This method, utilizing a clinical concept that malignant lesions are generally harder than normal surrounding tissues, has been used to examine several organs, such as the thyroid, liver, pancreas, prostate, and breast [4567]. Among the several types of elastography, shear-wave elastography (SWE) has been developed to measure quantitative elasticity values using the acoustic radiation force induced by the ultrasound beam itself [58]. The acoustic radiation force induces mechanical waves, including shear waves, which propagate transversely through the tissues [9,10]. The SWE image is displayed by mapping a color that represents the degree of elasticity in a conventional B-mode ultrasound image [4].
In this study, we aimed to determine whether SWE could be used to predict metastasis in excised axillary lymph nodes because the presence of metastatic foci increases the stiffness of lymph nodes via increased cell density (tumor cells are tightly packed), stromal, and cytoskeletal stiffness [3]. Several studies have examined whether SWE can predict nodal metastasis in preoperative evaluation in vivo [11121314] or ex vivo [1]. Thus, we evaluated the feasibility of using ex vivo SWE intraoperatively to predict metastatic involvement of excised lymph nodes in patients undergoing surgery for breast cancer.
A total of 63 patients who underwent mastectomy or breast-conserving surgery with axillary lymph node evaluation for breast cancer were prospectively enrolled in this study from May 2014 to April 2015. A total of 274 axillary lymph nodes obtained form 63 patients were evaluated using ex vivo SWE. Patients who received neoadjuvant chemotherapy (n=2) or whose SWE was performed poorly (n=6) were excluded. Finally, we analyzed a total of 228 lymph nodes excised from 55 patients; this included sentinel lymph nodes (SLNs), non-SLNs, and other axillary lymph nodes. Of these 55 patients, four patients were clinically lymph node-positive and six patients were diagnosed with axillary lymph node metastasis in preoperative fine needle aspiration. Two of the clinically lymph node-positive patients were also identified to have lymph node metastases by preoperative fine needle aspiration. SLN biopsy under the guidance of technetium 99mTc-labeled tin colloid detected by gamma probe and frozen section evaluation during the surgery were performed in clinically lymph node-negative patients. The lymph node with radioactive uptake equal to or greater than 10% of the highest radioactive lymph node was defined as the SLN. Axillary lymph node dissection was performed in patients who were clinically axillary lymph node-positive or with axillary lymph node metastasis confirmed by preoperative fine needle aspiration or intraoperative SLN biopsy. The metastatic status of the excised lymph nodes was confirmed with histological examination of the permanent pathological section. Nodal characteristics and elasticity values measured by ex vivo SWE were analyzed to determine whether they were correlated with nodal metastasis.
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Informed consent was obtained, and ethics approval for this study was granted by the Institutional Review Board of Yonsei University, Seoul, Republic of Korea (IRB number: 3-2015-0017).
The excised axillary lymph nodes were sent to the ultrasound room before the pathologic evaluation of the frozen sections. B-mode ultrasound and SWE examinations using the Aixplorer ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) and its ShearWave™ elastography mode were performed independently by two expert radiologists using a 4–15-MHz linear transducer. During the B-mode ultrasound and SWE examination, the radiologist needs to handle the excised lymph nodes carefully so as not to exert pressure on them with the ultrasound transducer. First, the specimen was examined with a B-mode ultrasound using a sufficient amount of contact jelly to avoid artefactual stiffness. The diameter of the axillary lymph node that appeared to be hypoechoic compared with the adjacent fat tissue was measured (Supplementary Figure 1A, available online). Then, the SWE images of at least two longitudinal planes was obtained. The region-of-interest (ROI) box (Q-Box™; SuperSonic Imagine) of the system was drawn to include the lesion and surrounding normal tissue. Overlaid on the gray-scale image was a semi-transparent color map of tissue stiffness with a range from dark blue, indicating the lowest stiffness, to red, indicating the highest stiffness (0–180 kPa). Similar to the appearance of malignant breast lesions during SWE, metastatic lymph nodes show the “stiff rim sign,” which is increased stiffness at the lesion's margin and adjacent tissue on the color elastic map. After few seconds of immobilization to allow the SWE image to stabilize, elasticity values (maximum and mean stiffness values [kPa]) were measured by placing a fixed 2-mm circular ROI on the stiffest region of the excised axillary lymph node [15]. The ratio of elasticity of lymph nodes to that of the adjacent subcutaneous adipose tissue was calculated automatically when the first ROI was placed on the stiffest region of excised lymph node, and the other ROI was immediately set over the adjacent fat tissue (Supplementary Figure 1B, available online). In addition, the imaging system automatically calculated the mean and maximum values of elasticity in kilopascals. Two radiologists analyzed the SWE images; both were blinded to the diagnosis and clinicopathologic factors.
The excised axillary lymph nodes were thinly sectioned (at 2–3 mm) and all slices were examined. The size of metastatic lesion within the excised node was reported according to the staging system of American Joint Committee on Cancer as follows: >2 mm, macro-metastasis; 0.2–2.0 mm, micro-metastasis; and <0.2 mm, isolated tumor cells [16].
The mean elasticity values were compared between nonmetastatic and metastatic nodes using the independent two sample t-test. Multivariable analysis was performed using binary logistic regression model adjusted for statistically significant factors in the univariable analysis. Correlation between elasticity values and nodal metastases were examined using Pearson correlation analysis. The Youden method was used to identify the cutoff values. Receiver operating characteristic curve (ROC) analysis of nodal size and elasticity values obtained via SWE for metastatic lymph nodes was performed. A p-value <0.05 was considered to indicate statistical significance, and 95% confidence intervals (CIs) are given. The software programs used to perform these analyses were SPSS version 20.0 (IBM Corp., Armonk, USA), SAS version 9.2 (SAS Institute, Cary, USA), and R package version 2.9.2.
A total of 274 lymph nodes, harvested from 63 breast cancer patients, were examined using ex vivo SWE. Of these 274 lymph nodes, 228 (from 55 patients) were eligible for our study. The median patient age was 49 years (range, 31–69 years). The median tumor size was 1.75 cm (range, 0.1–3.6 cm). Estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) were positive in 39 (70.9%) and 21 (38.2%) patients, respectively. The baseline clinicopathologic characteristics are presented in Table 1. Of the 228 lymph nodes, 187 (82.0%) were nonmetastatic and 41 (18.0%) were metastatic. Metastatic lymph nodes were significantly larger than nonmetastatic lymph nodes (mean size, 10.5 mm and 7.5 mm, respectively, p<0.001). The size of metastatic foci ranged from 3.0 mm to 21.5 mm with a mean of 9.3 mm (Supplementary Table 1, available online).
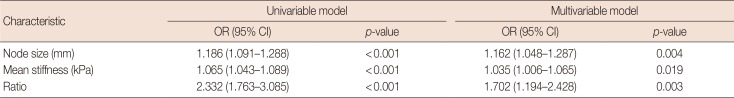
There was significant correlation between nodal metastasis and the elasticity values, including mean and maximum stiffness (kPa). The mean stiffness (45.4 kPa vs. 17.7 kPa) (Figure 1), maximum stiffness (55.3 kPa vs. 23.2 kPa), and elasticity ratio (4.36 vs. 1.57) of the metastatic lymph nodes were significantly higher than those of the nonmetastatic nodes. In the multivariate analysis, nodal size and elasticity values (mean stiffness and elasticity ratio) were associated with nodal metastasis (Table 2). In addition, the mean (r=0.553, p<0.001) and maximum (r=0.497, p=0.001) stiffness were significantly correlated with the size of the metastatic foci within the lymph nodes (Figure 2).
ROC curves and calibration plots were used to assess the discriminatory power of each elasticity value to identify nodal metastasis (Supplementary Figures 2 and 3, available online). The area under curve (AUC) for mean stiffness was 0.794 (95% CI, 0.710−0.879; p<0.001). The AUC values for the maximum stiffness and the elasticity ratio were 0.802 and 0.831, respectively (Figure 3A).
When the mean stiffness cutoff point of 30.6 kPa was used, the test showed high specificity (90.9%) and accuracy (85.1%) values. Other cutoff points were 8.9 mm for node size, 38.6 kPa for maximum stiffness, and 2.37 for the elasticity ratio (Supplementary Table 2, available online). When lymph nodes with micro-metastases were regarded as metastatic nodes, the AUC values for mean stiffness, maximum stiffness, and the elasticity ratio were 0.774, 0.786, and 0.809, respectively (Figure 3B).
Axillary lymph node metastasis is an important factor in the prognosis of patients with breast cancer. For this reason, SLN biopsy and frozen section evaluation is performed to assess the metastatic status of axillary lymph nodes during the breast cancer surgery; axillary lymph node dissection is performed if the SLNs harbor metastatic foci. However, the evaluation of intraoperative frozen section of SLN requires a pathologist with breast cancer expertise capable of assessing frozen sections; this prolongs the operation time and increases the total costs. We tested our hypothesis that ex vivo SWE is a reliable method to predict the presence of metastasis in excised axillary lymph node.
Since the development of elastography by Ophir and colleagues in 1990, its efficacy has been observed across several clinical fields, including disorders related to the thyroid, prostate, kidney, and pancreas, and deep venous thrombosis [517]. In terms of breast pathology, many studies have been conducted to determine the usefulness of elastography in the diagnosis of various breast lesions; in this context, elastography is widely used in clinical practice [181920]. These studies have revealed that the elasticity values of malignant lesions are higher than those of benign lesions. Moreover, the accuracy of the diagnosis is improved when SWE is added to conventional ultrasonography. In addition, studies on elastography for predicting axillary lymph node metastasis preoperatively have demonstrated its advantage of being a noninvasiveness method.
In our study, we assessed the elasticity values (mean stiffness, maximum stiffness, and elasticity ratio) of excised axillary lymph nodes using ex vivo SWE. The metastatic lymph nodes were larger, had higher values of mean and maximum stiffness, and a higher elasticity ratio than nonmetastatic lymph nodes. The mean sizes of metastatic and nonmetastatic lymph nodes were 10.5 mm and 7.5 mm. These findings were similar to findings in prior study that reported that metastatic lymph nodes were longer in the short and long axis and had a greater cortical thickness than nonmetastatic lymph nodes [1]. In contrast, another study reported no significant difference in the size of malignant and nonmalignant lymph nodes [11]. However, this study included only 11 metastatic lymph nodes and their size was relatively small (1.0–11.0 mm).
In addition, both the mean and maximum stiffness of the metastatic lymph nodes were higher than that of the nonmetastatic lymph nodes. Tourasse et al. [11] reported mean stiffness values of 17.5 and 11.3 kPa for metastatic and nonmetastatic axillary lymph nodes, respectively, when the lymph nodes were assessed using in vivo SWE preoperatively. Kilic et al. [1] reported mean stiffness values of metastatic and nonmetastatic axillary lymph nodes of 25.5 kPa and 10.7 kPa, respectively, when the lymph nodes were assessed by ex vivo SWE intraoperatively. In our study, the mean and maximum stiffness were higher than those previously reported. This is probably because we included 41 metastatic lymph nodes, whereas the authors included only 10s of nodes in the previous studies. The AUC values were 0.794 for mean stiffness and 0.802 for maximum stiffness. These were higher than the values of 0.762 for mean stiffness and 0.753 for maximum stiffness in an in vivo study; however, the mean stiffness is similar to the value of 0.786 for mean stiffness in an ex vivo study. Among the elasticity values, the elasticity ratio showed the highest value with an odds ratio of 2.332, and an AUC of 0.831. Several studies have reported that the elasticity ratio gives the best diagnostic performance in the evaluation of breast lesions by SWE [721]. This is because the elasticity ratio is the value least affected by pre-compression [22]. Moreover, our elasticity values may be more accurate than those obtained in an in vivo study because pre-compression is lower when ex vivo SWE is performed.
There are some limitations in our study. The greatest disadvantage of ultrasonography is its limited reproducibility [23]. However, when both B-mode ultrasound and SWE are applied for evaluating lesions, intraobserver and interobserver reproducibility of SWE is highly reliable for quantitative and qualitative assessment [24]. In a previous study, the intraobserver reliability of node diameter was highly acceptable (intra-class correlation coefficient [ICC], 0.94; 95% CI, 0.94−0.95) as it was for mean stiffness (ICC, 0.87; 95% CI, 0.85−0.88) and the elasticity ratio (ICC, 0.77; 95% CI, 0.74−0.79) of lymph nodes to the adjacent adipose tissue [24]. In addition, substantial interobserver agreement was noted for SWE homogeneity (κ=0.66) and for maximum elasticity (κ=0.80). The reproducibility of ultrasonographic examination, unlike that of in vivo examination of a breast mass, was likely to be higher in our study because we examined the ex vivo lymph nodes using better shear wave propagation; this allows better elastographic examination of the lymph nodes. In addition, our values of elasticity would be reliable because the SWE was performed by experienced radiologists. Another important limitation was that we did not count the exact time taken for intraoperative ex vivo SWE. Further research to investigate the effect of procedure on operation time is warranted. However, we expect that ex vivo SWE can be performed more rapidly than conventional frozen section evaluation of axillary lymph nodes due to the convenience of ex vivo SWE.
Despite these limitations, the surgeons could utilize intraoperative ex vivo SWE to evaluate the metastatic status of excised lymph nodes under the guidance of radiologists because B-mode and SWE examination is easy to perform and does not require any additional instruments or agents apart from an ultrasound machine with an on-board elastic mode [9].
A validation study is needed to determine the usefulness of ex vivo SWE to intraoperatively predict metastasis of actual SLNs. If ex vivo SWE can predict the presence the metastasis in SLN as well as frozen examination, then the former technique can potentially be an alternative intraoperative method to evaluate SLNs. In conclusion, the ex vivo SWE showed that the larger size and the higher elasticity values, such as mean, maximum stiffness, and ratio, were significantly correlated with the presence of metastasis in axillary lymph nodes. It suggested that the intraoperative ex vivo SWE can be a feasible method to predict the presence of metastasis in axillary lymph nodes.
Notes
References
1. Kilic F, Velidedeoglu M, Ozturk T, Kandemirli SG, Dikici AS, Er ME, et al. Ex vivo assessment of sentinel lymph nodes in breast cancer using shear wave elastography. J Ultrasound Med. 2016; 35:271–277. PMID: 26715659.

2. Garra BS. Elastography: current status, future prospects, and making it work for you. Ultrasound Q. 2011; 27:177–186. PMID: 21873855.
3. Faruk T, Islam MK, Arefin S, Haq MZ. The journey of elastography: background, current status, and future possibilities in breast cancer diagnosis. Clin Breast Cancer. 2015; 15:313–324. PMID: 25858446.

4. Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014; 33:149–160. PMID: 25038804.

5. Anvari A, Barr RG, Dhyani M, Samir AE. Clinical application of sonoelastography in thyroid, prostate, kidney, pancreas, and deep venous thrombosis. Abdom Imaging. 2015; 40:709–722. PMID: 25750099.

6. Youk JH, Son EJ, Park AY, Kim JA. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography. 2014; 33:34–39. PMID: 24936493.
7. Youk JH, Gweon HM, Son EJ, Han KH, Kim JA. Diagnostic value of commercially available shear-wave elastography for breast cancers: integration into BI-RADS classification with subcategories of category 4. Eur Radiol. 2013; 23:2695–2704. PMID: 23652850.

8. Barr RG, Zhang Z. Shear-wave elastography of the breast: value of a quality measure and comparison with strain elastography. Radiology. 2015; 275:45–53. PMID: 25426770.

9. Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, et al. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography. 2014; 33:3–10. PMID: 24936489.

10. Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, et al. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res. 2010; 12:R104. PMID: 21122101.

11. Tourasse C, Dénier JF, Awada A, Gratadour AC, Nessah-Bousquet K, Gay J. Elastography in the assessment of sentinel lymph nodes prior to dissection. Eur J Radiol. 2012; 81:3154–3159. PMID: 22656671.

12. Taylor K, O'Keeffe S, Britton PD, Wallis MG, Treece GM, Housden J, et al. Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: a pilot study. Clin Radiol. 2011; 66:1064–1071. PMID: 21835398.

13. Evans A, Rauchhaus P, Whelehan P, Thomson K, Purdie CA, Jordan LB, et al. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer. Breast Cancer Res Treat. 2014; 143:153–157. PMID: 24305976.

14. Choi JJ, Kang BJ, Kim SH, Lee JH, Jeong SH, Yim HW, et al. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J Ultrasound Med. 2011; 30:429–436. PMID: 21460142.

15. Skerl K, Vinnicombe S, Giannotti E, Thomson K, Evans A. Influence of region of interest size and ultrasound lesion size on the performance of 2D shear wave elastography (SWE) in solid breast masses. Clin Radiol. 2015; 70:1421–1427. PMID: 26455652.

16. Dabbs DJ, Fung M, Landsittel D, McManus K, Johnson R. Sentinel lymph node micrometastasis as a predictor of axillary tumor burden. Breast J. 2004; 10:101–105. PMID: 15009035.

17. Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991; 13:111–134. PMID: 1858217.

18. Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015; 41:1126–1147. PMID: 25805059.

19. Goddi A, Bonardi M, Alessi S. Breast elastography: a literature review. J Ultrasound. 2012; 15:192–198. PMID: 23449849.

20. Cho N, Moon WK, Park JS, Cha JH, Jang M, Seong MH. Nonpalpable breast masses: evaluation by US elastography. Korean J Radiol. 2008; 9:111–118. PMID: 18385557.

21. Au FW, Ghai S, Moshonov H, Kahn H, Brennan C, Dua H, et al. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. AJR Am J Roentgenol. 2014; 203:W328–W336. PMID: 25148191.

22. Lee BE, Chung J, Cha ES, Lee JE, Kim JH. Role of shear-wave elastography (SWE) in complex cystic and solid breast lesions in comparison with conventional ultrasound. Eur J Radiol. 2015; 84:1236–1241. PMID: 25937525.

23. Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012; 262:435–449. PMID: 22282182.

24. Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, et al. Shear wave elastography for breast masses is highly reproducible. Eur Radiol. 2012; 22:1023–1032. PMID: 22210408.

SUPPLEMENTARY MATERIALS
Supplementary Table 1
Comparison of ex vivo SWE values and characteristics between nonmetastatic and metastatic axillary lymph nodes
Supplementary Table 2
Diagnostic value of SWE values, including mean stiffness, node size, max stiffness, and ratio
Supplementary Figure 1
B-mode ultrasound and shear-wave elastography (SWE). (A) B-mode ultrasound showed 1.72-cm sized excised axillary lymph node with adjacent fat tissue. (B) The mean and max stiffness were measured by placing the 2-mm sized region-of-interest (ROI) over the stiffest part of the excised axillary lymph node (circle). The elasticity ratio of the axillary lymph node to the adjacent fat tissue was measured by placing another ROI on the surrounding fat tissue (dotted circle).
Supplementary Figure 2
The calibration plot showed good agreement between the predicted and observed probabilities. (A) node size, (B) mean stiffness, (C) max stiffness, and (D) elasticity ratio of nonmetastatic and metastatic nodes.
Supplementary Figure 3
The calibration plot showed good agreement between the predicted and observed probabilities. (A) node size, (B) mean stiffness, (C) max stiffness, and (D) elasticity ratio of lymph nodes when micro-metastasis is regarded as metastatic nodes.
Figure 1
Characteristics of metastatic and nonmetastatic lymph node. Box plot showing size (A), mean stiffness (B), max stiffness (C), and the elasticity ratio (D) between nonmetastatic and metastatic lymph nodes.
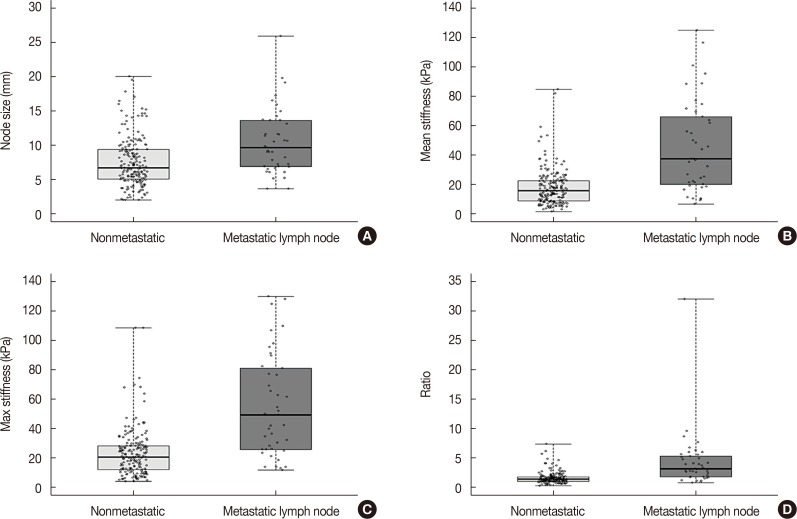
Figure 2
Correlation coefficients for metastatic node size and ex vivo shear-wave elastic values. (A) Mean stiffness, (B) max stiffness.
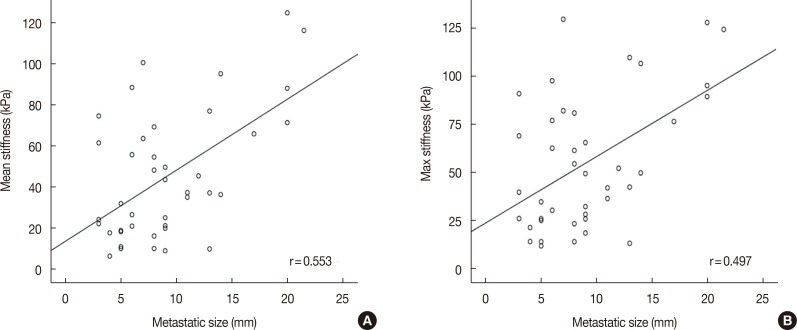
Figure 3
Receiver operating characteristic (ROC) curve analysis of size, mean stiffness, max stiffness, and elasticity ratio. (A) Lymph nodes with micro-metastasis were regarded as nonmetastatic nodes, (B) lymph nodes with micro-metastasis were regarded as metastatic nodes.
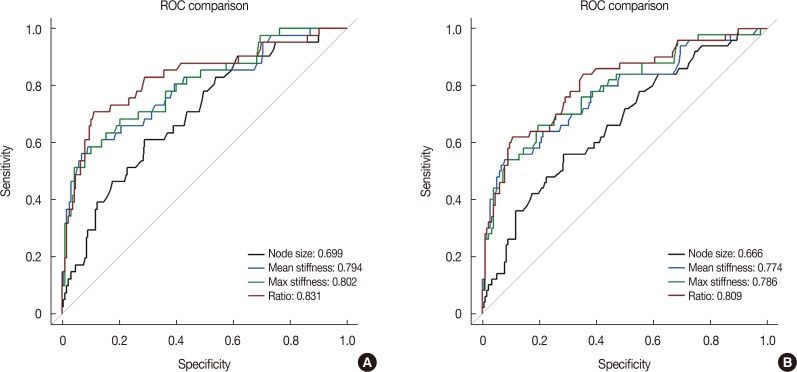
Table 1
Clinicopathologic characteristics of the 55 patients
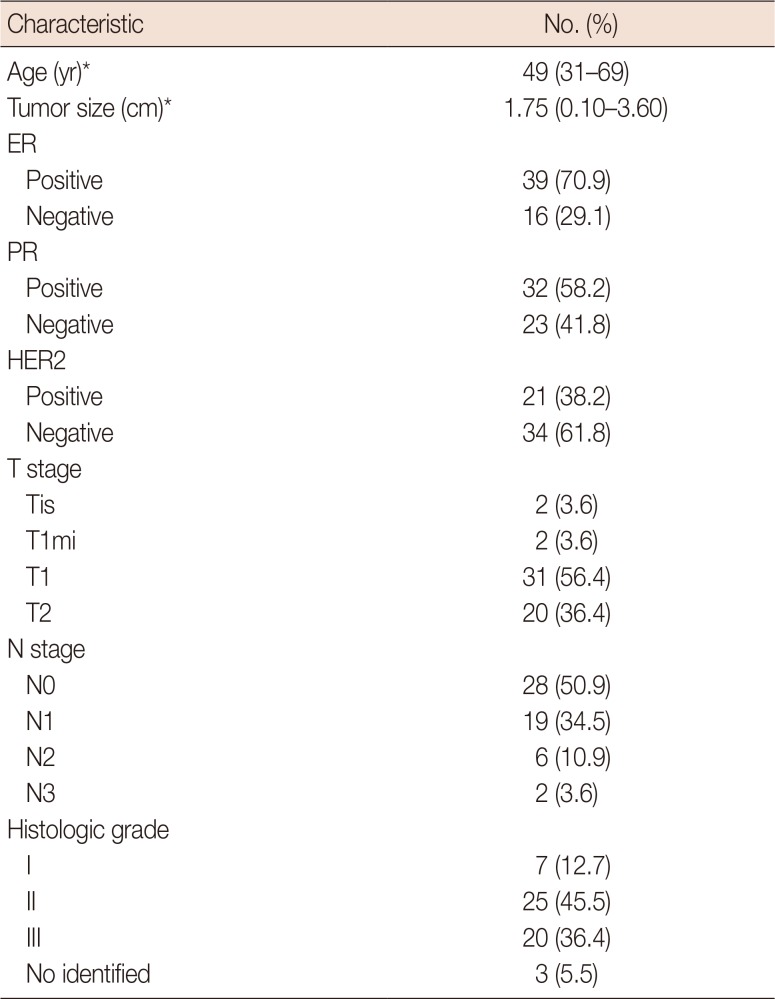
Table 2
Ex vivo SWE values and characteristics between nonmetastatic and metastatic axillary lymph nodes in uni- and multivariable models




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download