Abstract
Purpose
We evaluated the utility of magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) for the preoperative staging of invasive lobular carcinoma (ILC) of the breast and compared the results with those of invasive ductal carcinoma (IDC).
Methods
The study included pathologically proven 32 ILCs and 73 IDCs. We compared clinical and histopathological characteristics and the diagnostic performances of MRI and 18F-FDG PET/CT for the primary mass, additional ipsilateral and/or contralateral lesion(s), and axillary lymph node metastasis between the ILC and IDC groups.
Results
Primary ILCs were greater in size, but demonstrated lower maximum standardized uptake values than IDCs. All primary masses were detected on MRI. The detection rate for ILCs (75.0%) was lower than that for IDCs (83.6%) on 18F-FDG PET/CT, but the difference was not significant. For additional ipsilateral lesion(s), the sensitivities and specificities of MRI were 87.5% and 58.3% for ILC and 100.0% and 66.7% for IDC, respectively; whereas the sensitivities and specificities of 18F-FDG PET/CT were 0% and 91.7% for ILC and 37.5% and 94.7% for IDC, respectively. The sensitivity of 18F-FDG PET/CT for ipsilateral lesion(s) was significantly lower in the ILC group than the IDC group. The sensitivity for ipsilateral lesion(s) was significantly higher with MRI; however, specificity was higher with 18F-FDG PET/CT in both tumor groups. There was no significant difference in the diagnostic performance for additional contralateral lesion(s) or axillary lymph node metastasis on MRI or 18F-FDG PET/CT for ILC versus IDC.
Conclusion
The MRI and 18F-FDG PET/CT detection rates for the primary cancer do not differ between the ILC and IDC groups. Although 18F-FDG PET/CT demonstrates lower sensitivity for primary and additional ipsilateral lesions, it shows higher specificity for additional ipsilateral lesions, and could play a complementary role in the staging of ILC as well as IDC.
Invasive lobular carcinoma (ILC) is the second most common breast cancer after invasive ductal carcinoma (IDC), accounting for 5% to 20% of all breast cancers [1,2,3]. The increase in incidence of ILC is strongly associated with hormone replacement therapy [4]. ILC is difficult to detect upon physical examination and the diagnostic performances of mammography [5,6] and ultrasonography (US) [7,8] for ILC are lower than for IDC. Moreover, ILC is more often multifocal and multicentric [9,10] as well as bilateral [11]. Both mammography and US tend to underestimate lesion size and miss multifocal or multicentric lesions [2,3,5,12].
The role of magnetic resonance imaging (MRI) in the preoperative staging workup of ILC has been reported in several studies [2,3,9,13]. Contrast-enhanced MRI has been shown to be more accurate in terms of preoperative staging and determining the extent of ILC than mammography or US. MRI can also detect additional ipsilateral and contralateral lesions, and often leads to changes in patient management [3,9,13].
Although the value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) for staging primary breast cancer has not yet been established, recent studies have shown its potential for the preoperative staging of breast cancer [14,15]. However, the sensitivities of 18F-FDG PET/CT for primary cancer, multiple lesions, and axillary lymph node metastasis are not comparable to those of US or MRI. Its use is limited to applications requiring a high positive predictive value and the detection of extra-axillary nodal or distant metastasis. Furthermore, ILCs are known to show low 18F-FDG uptake and high false-negative rates on 18F-FDG PET/CT [16,17], so its value in the preoperative staging of ILC is more challenging.
Few studies have compared the efficacy of 18F-FDG PET/CT in the preoperative staging of breast cancer with that of MRI for ILC versus IDC. The aim of this study was to evaluate the value of breast MRI and 18F-FDG PET/CT in the preoperative staging of ILC of the breast and to compare the results with those of IDC.
Institutional Review Board approval was obtained for this retrospective study (IRB number: KC12RISI07854). The need for informed consent was waived.
We reviewed medical records and identified 35 patients with a surgical diagnosis of ILC between January 2004 and June 2012 and 85 patients with a surgical diagnosis of IDC in 2009, exclusive of a case of mixed lobular and ductal carcinoma (n=1) and cases of IDC with an extensive intraductal component (n=2). All patients underwent preoperative MRI. We excluded 15 patients (3 ILC and 12 IDC) without 18F-FDG PET/CT results. Thus, a total of 105 patients (32 ILC and 73 IDC) were included in the study.
MRI scans were acquired with the patient in a prone position in a 1.5-T scanner (Achieva; Philips Medical Systems, Best, The Netherlands) or a 3.0-T scanner (Magnetom Verio; Siemens Medical Solutions, Erlangen, Germany) equipped with a breast coil. MRI images from the Achieva scanner used the following sequences: a sagittal, fat-suppressed, fast spin-echo T2-weighted imaging sequence (repetition time/echo time [TR/TE], 6,000/100 ms; flip angle, 90°; 30 slices; field of view [FOV], 320 mm; matrix, 424×296; number of excitations [NEX], 1; 4-mm slice thickness; 0.1-mm interslice gap; and an acquisition time of 2 minutes 56 seconds) and pre- and postcontrast dynamic axial T1-weighted three-dimensional, fatsuppressed, fat-spoiled gradient-echo sequences (TR/TE, 6.9/3.4 ms; flip angle, 12°; 2.0-mm slice thickness; and an acquisition time of 1 minute 31 seconds) obtained before and at 91, 182, 273, 364, and 455 seconds after a rapid bolus injection of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) (Magnevist; Schering, Berlin, Germany) at 0.2 mmol/kg body weight. MRI images from the Magnetom Verio scanner were acquired using the following sequences: an axial, turbo spin-echo T2-weighted imaging sequence (TR/TE, 4,530/93 ms; flip angle, 80°; 34 slices; FOV, 320 mm; matrix, 576×403; NEX, 1; 4-mm slice thickness; and an acquisition time of 2 minutes 28 seconds) and pre- and postcontrast axial T1-weighted fast low-angle shot three-dimensional, volumetric interpolated breath-hold examination sequences (TR/TE, 4.4/1.7 ms; flip angle, 10°; 1.2-mm slice thickness; and an acquisition time of 7 minutes 7 seconds) obtained before and at 7, 67, 127, 187, 247, and 367 seconds after Gd-DTPA injection.
All patients underwent preoperative 18F-FDG PET/CT examinations using a dedicated PET/CT scanner with 2-slice CT (Siemens Biograph Classic; Siemens Medical Solutions, Knoxville, USA) (n=60) or a PET/CT scanner with 40-slice CT (Siemens Biograph TruePoint; Siemens Medical Solutions) (n=45). The patients fasted for at least 6 hours before the examination. Serum glucose levels were measured to ensure euglycemia (blood glucose level <130 mg/dL). Then, 370 to 550 MBq of 18F-FDG was injected with a saline infusion. After 60 minutes of postinjection bed rest, PET scans were performed. Seven or eight bed positions were acquired with an acquisition time of 2 minutes each. All patients were in a supine position with their arms raised during PET/CT scanning. Noncontrast CT scanning began at the orbitomeatal line and progressed to the upper thigh (30 mAs, 130 kVp, and a 5-mm slice thickness) and corresponding PET imaging followed immediately over the same body region. The CT data were used for attenuation correction and anatomic localization of lesions.
The MRI data were interpreted retrospectively by two dedicated breast radiologists with 6 and 7 years of experience in breast imaging who were blinded to previous imaging and histopathological results. Any difference in opinion was resolved by consensus between the radiologists. The readers evaluated any enhancing lesion by morphological and kinetic features according to the American College of Radiology Breast Imaging-Reporting and Data System MR lexicon [18]. All patients underwent percutaneous biopsies for suspicious lesions found on previous mammography and US before preoperative MRI for staging. The percutaneous biopsy method was a US-guided 14-gauge automated core biopsy in all cases. The pathologically proven index lesion was considered the primary mass. Additional suspicious malignant lesions were categorized as ipsilateral or contralateral lesions and compared with the location of the primary mass. Suspicious MRI findings were defined as follows: a spiculated or irregular mass with heterogeneous or rim enhancement; non-mass-like enhancement with ductal, segmental, or regional distribution; and enhancement with a washout kinetic curve pattern [11,12,19,20]. Any enlarged or rounded axillary lymph node with cortical thickening or loss of fatty hilum was considered to be a metastatic lymph node [18].
The 18F-FDG PET/CT imaging was interpreted retrospectively by two nuclear medicine physicians with 4 and 5 years of experience. All PET/CT images were reviewed at a workstation using the Fusion software program (Syngo; Siemens Medical Solutions), which provided multiplanar reformatted images and displayed PET images, CT images, and PET/CT fusion images. Any difference in opinion was resolved by consensus between the physicians. They reviewed and interpreted the PET/CT images by visual assessment. Any lesion with 18F-FDG uptake greater than the background parenchymal uptake at the site of a pathologically proven malignancy was defined as a primary tumor. For a semiquantitative analysis, the maximum standardized uptake value (SUVmax) of 18F-FDG was measured by placing regions of interest around primary cancer masses that had perceptible 18F-FDG uptake. Any additional foci detected visually from increased 18F-FDG uptake that exceeded the background parenchymal uptake were recorded and categorized as ipsilateral or contralateral findings. If 18F-FDG uptake was perceptible in an axillary lymph node, the case was deemed positive for axillary lymph node metastasis. Conversely, if no 18F-FDG uptake was perceptible in the axillary regions, the case was deemed free of axillary lymph node metastasis. To compensate any qualitative disparity between two different PET/CT scanner types, mean liver SUV values were obtained for all patients. The tumor/liver SUV ratio was calculated and compared between the two different PET/CT scanner types. The tumor/liver SUV ratio was not statistically different according to the PET/CT scanner type (2.11±1.89 for the Biograph Classic with 2-slice CT vs. 1.75±1.26 for the Biograph TruePoint with 40-slice CT, p=0.299).
The surgical pathological results were used as the reference standard to evaluate the diagnostic performance of MRI and 18F-FDG PET/CT for the detection of the primary cancer, additional ipsilateral and/or contralateral lesion(s), and axillary lymph node metastasis. Additional findings with a pathologically proven malignancy were considered true positives. Additional findings with a pathologically proven high-risk lesion such as lobular carcinoma in situ (LCIS) or benign pathology were considered false positives. If a tissue diagnosis was unattainable, follow-up studies were used to evaluate the clinical significance of additional findings on MRI and/or 18F-FDG PET/CT. We obtained information about the primary cancer, including tumor size and grade, from the pathology report.
Continuous variables are shown as means±standard deviations and categorical variables are presented as frequencies and percentages. Differences in patient age, tumor size, and SUVmax between the ILC and IDC groups were compared using the Wilcoxon rank-sum test. Differences in tumor grade and frequency of additional ipsilateral and/or contralateral lesion(s) and axillary lymph node metastasis between the ILC and IDC groups were compared using the chi-square test and Fisher exact test. The correlation between tumor size and SUVmax was assessed using Spearman correlation coefficient and a p-value.
For the primary cancer, the detection rates of ILC and IDC are presented as percentages and probabilities (95% confidence interval) and compared using the chi-square test and Fisher exact test. The SUVmax cutoff values for the semiquantitative assessment of 18F-FDG PET/CT were set at 2.5, 2, 1.5, and 1. For additional ipsilateral and/or contralateral lesion(s) and axillary lymph node metastasis, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and area under the curve (AUC) from a receiver operating characteristic (ROC) curve analysis were obtained for the ILC and IDC groups on MRI and PET/CT, respectively. Diagnostic performances were compared using the chi-square test and Fisher exact test. Statistical significance was set at p<0.05. All evaluations were performed using SAS software version 9.1 for Windows (SAS Institute, Cary, USA).
The clinical and histopathological data are summarized in Table 1. Tumor size was significantly greater in the ILC group (2.64±1.13 cm) than in the IDC group (2.08±1.69 cm, p=0.002). The mean SUVmax in the ILC group (1.99±1.72) was significantly lower than that in the IDC group (3.91±3.99, p=0.032). Patient age, tumor grade, and the frequency of additional ipsilateral and/or contralateral lesion(s) and axillary lymph node metastasis did not differ between the ILC and IDC groups. Tumor size and SUVmax were correlated in the IDC group, but not in the ILC group. Spearman correlation coefficients for tumor size and SUVmax were 0.25 for ILC (p=0.179) and 0.57 for IDC (p<0.001). Mastectomy was performed in 53 patients (50.5%), breast-conserving surgery in 50 patients (47.6%), and mastectomy with contralateral breast-conserving surgery in two patients (1.9%).
The detection rate for the primary breast cancer is presented in Table 2. With MRI, all primary ILC and IDC masses were detected. With 18F-FDG PET/CT, the detection rate for ILC (75.0%) was lower than that for IDC (83.6%), but did not achieve statistical significance. In both tumor groups, the detection rate on MRI was significantly higher than on 18F-FDG PET/CT. The detection rate for the visual assessment of PET/CT was higher than that of a semiquantitative analysis using SUVmax cutoff values of 2.5, 2, 1.5, or 1 (Figure 1). The detection rate using a semiquantitative assessment increased significantly as the cutoff value was lowered.
From the surgical pathology, additional ipsilateral lesion(s), contralateral lesion(s), and axillary lymph node metastasis were noted in 25.0% (8/32), 3.1% (1/32), and 31.3% (10/32) of ILC patients and in 21.9% (16/73), 1.4% (1/73), and 37.0% (27/73) of IDC patients, respectively (Table 1). The diagnostic performances of MRI and 18F-FDG PET/CT for additional ipsilateral and contralateral lesion(s) and axillary lymph node metastasis are presented in Table 3.
Additional suspicious ipsilateral finding(s) were detected in 52 cases (17 ILC and 35 IDC) on MRI, and 11 (2 ILC and 9 IDC) of these 52 cases were also demonstrated on 18F-FDG PET/CT. No additional suspicious ipsilateral finding was detected exclusively on 18F-FDG PET/CT. A pathological correlation was performed in 51 cases by sonographically guided core-needle biopsy (n=10, 4 ILC and 6 IDC), sonographically guided localization for excisional biopsy (n=16, 6 ILC and 10 IDC), mammographically guided localization for excisional biopsy (n=1, 1 IDC), and by checking the surgical specimen for the primary cancer, which was from a mastectomy (n=22, 5 ILC and 17 IDC) or wide excision (n=2, 2 ILC). The one remaining suspicious finding (1 IDC) was not seen on second look US examination and was followed postoperatively by mammography, US, and PET/CT for 5 years without evidence of any suspicious lesion. Twenty-four out of 52 cases (8/17 for ILC and 16/35 for IDC) proved to be true-positive additional ipsilateral lesions.
Comparing 18F-FDG PET/CT between the ILC and IDC groups, the sensitivity for ipsilateral lesion(s) was significantly lower in the ILC group than the IDC group (0% vs. 37.5%, p=0.046). The sensitivity, specificity, and accuracy of MRI and the specificity and accuracy of 18F-FDG PET/CT were not significantly different between the ILC and IDC groups.
Comparing the diagnostic performance between MRI and 18F-FDG PET/CT in the ILC group, the sensitivity was significantly higher with MRI (87.5% vs. 0%, p=0.001); however, the specificity was higher with 18F-FDG PET/CT (58.3% vs. 91.7%, p=0.008) (Figure 2). The accuracy for ipsilateral lesion(s) in the ILC group was not significantly different between MRI and 18F-FDG PET/CT (65.6% vs. 68.8%, p=0.790). The AUC from the ROC analysis was significantly higher for MRI than 18F-FDG PET-CT (0.73 vs. 0.54, p=0.027).
Comparing the diagnostic performance between MRI and 18F-FDG PET/CT in the IDC group, the sensitivity was significantly higher with MRI (100.0% vs. 37.5%, p<0.001) (Figure 3); however, the specificity was higher with 18F-FDG PET/CT (66.7% vs. 94.7%, p<0.001). The accuracy for ipsilateral lesion(s) in the IDC group was not significantly different (74.0% vs. 82.2%, p=0.230). The AUC from the ROC analysis was significantly higher for MRI than 18F-FDG PET-CT (0.83 vs. 0.66, p=0.008).
MRI detected nine additional suspicious contralateral findings (3 ILC and 6 IDC), three of which (1 ILC and 2 IDC) were also detected on 18F-FDG PET/CT. Seven of the nine additional suspicious contralateral findings on MRI were correlated pathologically by sonographically guided core-needle biopsy (n=6, 3 ILC and 3 IDC) and sonographically guided localization for excisional biopsy (n=1, 1 IDC). The two remaining additional suspicious findings were not seen on second-look US examination and were followed postoperatively by mammography, US, and PET/CT for 5 years. 18F-FDG PET/CT detected two (2 IDC) additional suspicious contralateral findings not seen on MRI. One was also seen on US, and was confirmed by sonographically guided core-needle biopsy. However, the other suspicious finding was not seen on mammography or US, and was followed postoperatively on PET/CT after 12 and 41 months. This finding was not visible on either follow-up PET/CT examination. Two out of nine cases (1/3 for ILC, 1/6 for IDC) detected on MRI proved to be true-positive additional contralateral lesions. Neither of the two cases detected exclusively on 18F-FDG PET/CT proved to be malignant. Axillary lymph node metastasis was confirmed pathologically by axillary lymph node dissection (n=57, 16 ILC and 41 IDC) or sentinel lymph node biopsy (n=48, 16 ILC and 32 IDC).
The diagnostic performance of MRI and 18F-FDG PET/CT for additional contralateral lesion(s) and axillary lymph node metastasis was not significantly different between the ILC and IDC groups. There was no statistically significant difference between the diagnostic performances of MRI and 18F-FDG PET/CT in the detection of contralateral lesion(s) and axillary lymph node metastasis in either cancer group. In the ILC case with an additional contralateral lesion, 18F-FDG PET/CT could not detect the primary ILC, but could demonstrate the contralateral IDC (Figure 4).
ILC tends to be greater in size than IDC because of its infiltrative growth pattern and lesser desmoplastic reaction, which hinder early detection on physical examination and conventional imaging modalities [4,21]. ILCs are known to have low 18F-FDG uptake and high false-negative rates on 18F-FDG PET/CT and PET [15,16,17]. The lower 18F-FDG uptake of ILC has been explained by its diffuse infiltrative growth patterns, low tumor cell density, low level of glucose transporter 1 expression, and decreased proliferation rate [16,17,22]. In the current study, the ILCs were larger in size and demonstrated lower SUVmax values than IDCs, consistent with previous reports [4,15,16,17,21,22]. A correlation between tumor size and SUVmax was demonstrated for IDC, but not ILC. This reflects that ILC could show a low SUV, despite a large tumor size.
The frequency of additional ipsilateral and/or contralateral lesion(s) has been reported to be higher in ILC than IDC [9,11,19]. In the current study, additional ipsilateral lesions were observed in 25.0% (8/32) of the ILC group versus 21.9% (16/73) of the IDC group, while additional contralateral lesions were observed in 3.1% (1/32) of the ILC group versus 1.4% (1/73) of the IDC group. However, there was no significant difference between the ILC and IDC groups. The incidence of axillary lymph node involvement in ILC was evaluated in previous reports [3,21,23]. Vandorpe et al. [23] demonstrated that lobular cancers had a lower incidence of axillary lymph node involvement in a multivariate analysis. In the current study, the incidence of axillary lymph node metastasis did not differ between the ILC and IDC groups.
The value of MRI in detecting primary breast cancer has been investigated, with the reported sensitivity ranging from 95% to 100% [2,3,24,25]; the sensitivity of MRI in the current study was 100.0% for both ILC and IDC, consistent with previous reports. Choi et al. [14] reported that the sensitivity of 18F-FDG PET/CT for primary breast cancer was 89.6%, which is similar or slightly higher than that of the IDC group in our study. This is probably related to the fact that most (141/154, 91.6%) cases in their study were IDC cases. Groves et al. [15] reported that 95% of IDC and 70% of ILC cases were identified using 18F-FDG PET/CT in their prospective study. In the current study, the sensitivity of 18F-FDG PET/CT for primary cancer was higher in the IDC group than the ILC group, but there was no statistically significant difference. In a semiquantitative assessment of 18F-FDG PET/CT, even when the cutoff value was set to 1.0, the cancer detection rate was lower than that of visual assessment. However, the sensitivity of 18F-FDG PET/CT was not as high as that of MRI, even though a more sensitive visual assessment was used for both IDC and ILC groups.
The high sensitivity of MRI for multiple additional lesions of ILC has been reported previously [1,3,9,10,13,24]. The reported specificity of MRI for additional breast cancer foci was 65% to 80%, which was lower than the sensitivity [10,26]. Stivalet et al. [1] suggested that suspicious MRI findings should be verified by US- or MRI-guided biopsy because of the high false-positive rate. Indeed, there are concerns about the high false-positive rate, overestimation of tumor extent on MRI, and resulting unnecessary mastectomies, but recent studies have shown that preoperative MRI can reduce the re-excision rate without increasing the rate of unnecessary mastectomies [13,25,27]. Few studies have evaluated the diagnostic performance of 18F-FDG PET/CT for multiple additional lesions. Choi et al. [14] reported that the sensitivity of 18F-FDG PET/CT for multiple additional lesions was lower than that of both MRI and US (12.5%, 80.0%, and 78.4%, respectively); however, the specificity was highest among the three modalities (99.1%, 92.1%, and 86.3%, respectively). They reported that they did not find a diagnostic role of 18F-FDG PET/CT in differentiating multiple tumors from a single tumor, with its low sensitivity of 12.5%. In the current study, the sensitivities of 18F-FDG PET/CT in detecting additional ipsilateral lesion(s) were 0.0% for ILC and 37.5% for IDC; the specificities were 91.7% and 94.7%, respectively. Although the diagnostic role of 18F-FDG PET/CT in detecting additional ipsilateral lesions is limited, especially in ILC, an ancillary role of the modality could be possible in practice. If additional suspicious ipsilateral findings on MRI cannot be found on 18F-FDG PET/CT, the suspicion for malignancy is lower than that for findings also found on 18F-FDG PET/CT. In these cases, we could perform second-look US examination rather than direct US- or MRI-guided biopsy as the next step, and then choose US-guided biopsy or postoperative follow-up according to the ultrasonographic findings.
In a previous meta-analysis by Mann et al. [3], unexpected cancer in the contralateral breast was identified exclusively by MRI in 7% of cases. In previous studies, the suspicious contralateral finding detection rates for contrast-enhanced MRI ranged from 24.0% (28/118) [28] to 32.0% (72/223) [11]. Among these populations, 18.6% (22/118) and 5.0% (12/223) were confirmed to have contralateral malignant lesions, respectively. To our knowledge, no previous study has evaluated the diagnostic performance of PET/CT or PET for contralateral breast lesion(s). In the current study, only two additional contralateral lesions were included, which were detected by both preoperative MRI and 18F-FDG PET/CT. The number of false-positive cases for multiple contralateral lesions was higher on MRI (n=7, 5 IDC and 2 ILC) than 18F-FDG PET/CT (n=3, 3 IDC). However, there was no statistically significant difference in diagnostic performance for the detection of contralateral lesions between MRI and 18F-FDG PET/CT.
MRI is known to be less sensitive for the detection of axillary lymph node metastasis [9]. The sensitivities of 18F-FDG PET and PET/CT were also not sufficiently high [29,30]. In their prospective study, Fehr et al. [29] reported that the sensitivity, specificity, PPV, and NPV of 18F-FDG PET for nodal status were 20%, 93%, 67%, and 62%, respectively. In their study, PET missed all micrometastases and the largest detectable lymph node metastasis was 10 mm. In a more recent study, Heusner et al. [30] reported that the sensitivity, specificity, PPV, NPV, and accuracy of 18F-FDG PET/CT were 58%, 92%, 82%, 77%, and 79%, respectively, in a patient-based analysis. In a lesion-based analysis, the best sensitivity was 41%, reflecting the incapability of 18F-FDG PET/CT to detect very small lymph node metastases and micrometastases. They concluded that 18F-FDG PET/CT cannot replace invasive approaches for axillary staging, but may extend the indications for sentinel lymph node biopsy because of its high specificity and accuracy. In a previous study [14], the sensitivity and specificity of 18F-FDG PET/CT for axillary lymph node metastasis were reported to be 37.3% and 95.8%, respectively. In the current study, the sensitivities of MRI and 18F-FDG PET/CT for detecting axillary lymph node metastasis were 50.0% and 60.0% for ILC and 48.2% and 40.7% for IDC, respectively; the specificities of MRI and 18F-FDG PET/CT were 81.8% and 72.7% for ILC and 82.6% and 80.4% for IDC, respectively. Although there was no significant difference, 18F-FDG PET/CT showed higher sensitivity for the detection of axillary lymph node metastasis than MRI in ILC. It is an inconsistent and interesting finding, considering its low sensitivity for primary and additional ipsilateral ILC lesions. The reliability and possible pathophysiology of this finding need to be investigated in future studies.
The main limitation of the current study was the small number of cases, especially for ILC. In addition, the reviewers were aware that all cases had breast cancer, although they were unaware if the cases were ILC or IDC. Thus, they paid particular attention to evaluating primary and additional unilateral and/or bilateral finding(s) both on MRI and by visual assessment of 18F-FDG PET/CT. 18F-FDG PET/CT was considered to be the standard for distant metastasis, but no case showing this ability of the modality was included in the study. Add itionally, for suspicious findings detected on MRI, we performed a second-look US examinations and examined US-detectable lesions pathologically by US-guided biopsy or localization in most cases (58/61). MR-guided breast biopsy was not performed. Additional MR-detected suspicious ipsilateral and/or contralateral finding(s) with no pathological confirmation (3/61) were followed postoperatively by mammography, US, and 18F-FDG PET/CT, but not by MRI, and were regarded as true negatives. Finally, determining the correlation between the exact location of a suspicious finding on MRI, suspicious 18F-FDG uptake on 18F-FDG PET/CT, and the true lesion in a pathological specimen was difficult in practice in some cases. There was a possibility of an underestimation of additional ipsilateral and contralateral malignant lesion(s) if the lesion was not suspected in preoperative imaging studies. Future studies that include a large number of ILC samples are needed to thoroughly examine mastectomy specimens in a prospective manner.
In conclusion, the detection rates of MRI and 18F-FDG PET/CT for the primary cancer do not differ between the ILC and IDC groups. MRI detects more primary cancers and additional ipsilateral lesions than 18F-FDG PET/CT in both the ILC and IDC groups. However, 18F-FDG PET/CT demonstrates higher specificity for additional ipsilateral lesions, which could compensate for the lower specificity of MRI. The diagnostic performance for additional contralateral lesion(s) and axillary lymph node metastasis do not differ significantly between MRI and 18F-FDG PET/CT in the ILC or IDC group.
Figures and Tables
Figure 1
A 40-year-old female with invasive lobular carcinoma with a low maximum standardized uptake value (SUVmax). (A) Axial contrast-enhanced magnetic resonance imaging shows a heterogeneously enhanced irregular mass with irregular margin (arrow) at right mid outer breast. Axial 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) fusion image (B) and PET-only image (C) show focal mild FDG uptake (arrows) in the right outer breast with a SUVmax measured at 1.0. The lesion was categorized as positive for a primary mass by visual assessment.

Figure 2
A 45-year-old female with invasive lobular carcinoma (ILC) with a false-positive ipsilateral lesion on magnetic resonance imaging (MRI). (A) Axial postcontrast MRI at the nipple level shows a heterogeneously enhancing mass (circle) in the right mid outer breast. (B) Axial postcontrast MRI at the level of 1.5 cm cranial to the nipple shows an additional smaller enhancing mass (circle) at the right upper outer breast. (C) Kinetic curve obtained at the mass in the right upper outer breast (in Figure 2B) shows early rapid enhancement and a delayed washout pattern. (D-G) Axial 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) fusion image and PETonly image at the nipple level (D, E) show ill-defined mild focal FDG uptake (arrows) in the right mid outer breast with maximum standardized uptake value measured at 2.1, with no additional FDG uptake cranially (F, G). After a wide excision with ultrasonographically guided wire localization, the primary mass was confirmed as ILC, but the additional lesion was confirmed as lobular carcinoma in situ.
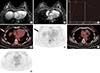
Figure 3
A 44-year-old female with invasive ductal carcinoma (IDC) with an additional ipsilateral lesion with true-positive on magnetic resonance imaging (MRI) and false-negative on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). (A) Axial contrast enhanced MRI shows a heterogeneously enhancing irregular mass with irregular margin (arrow) at right mid outer breast. Additionally, a smaller heterogeneously enhancing lesion was visible at right outer periareolar region (arrowhead). (B) Axial 18F-FDG PET/CT fusion image demonstrates strong FDG uptake (arrow) on primary tumor with a maximum standardized uptake value measured at 6.9. But no additional ipsilateral lesion on right outer periareolar region on 18F-FDG PET/CT fusion images at the level of primary mass (B) and nipple level (C). After breast-conserving surgery, the primary lesion was confirmed as IDC, and the additional lesion was confirmed as ductal carcinoma in situ.

Figure 4
A 48-year-old female with invasive lobular carcinoma in the left breast with a contralateral invasive ductal carcinoma in the right breast. (A) Axial postcontrast magnetic resonance imaging (MRI) at the left nipple level shows an indistinct irregular heterogeneously enhancing mass (arrow) in the left mid outer breast. (B) Axial postcontrast MRI of the right upper breast shows another indistinct irregular heterogeneously enhancing mass (arrow) in the right upper outer breast. (C) 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in the left mid outer breast shows little FDG uptake (arrow) in the corresponding area of the left breast mass. (D) 18F-FDG PET/CT shows ill-defined focal FDG uptake (arrow) in the right upper outer breast with a maximum standardized uptake value measured at 2.5.
Table 1
Clinical and histopathological characteristics of ILC and IDC patients

Table 2
Detection rate of primary breast cancer: MRI and PET/CT at different cutoff values of SUVmax

Table 3
The diagnostic performance of MRI and PET/CT for the ILC and IDC groups for additional ipsilateral and contralateral lesion(s) and axillary lymph node metastasis

MRI=magnetic resonance imaging; PET/CT=positron emission tomography/computed tomography; ILC=invasive lobular carcinoma; IDC=invasive ductal carcinoma; CI=confidence interval.
*(+)=number of total positive cases for additional ipsilateral, contralateral lesions(s), or axillary lymph node metastasis; number of positive ILC cases, number of positive IDC cases.
Notes
References
1. Stivalet A, Luciani A, Pigneur F, Dao TH, Beaussart P, Merabet Z, et al. Invasive lobular carcinoma of the breast: MRI pathological correlation following bilateral total mastectomy. Acta Radiol. 2012; 53:367–375.

2. Boetes C, Veltman J, van Die L, Bult P, Wobbes T, Barentsz JO. The role of MRI in invasive lobular carcinoma. Breast Cancer Res Treat. 2004; 86:31–37.

3. Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008; 107:1–14.

4. Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003; 289:1421–1424.

5. Veltman J, Boetes C, van Die L, Bult P, Blickman JG, Barentsz JO. Mammographic detection and staging of invasive lobular carcinoma. Clin Imaging. 2006; 30:94–98.

6. Hilleren DJ, Andersson IT, Lindholm K, Linnell FS. Invasive lobular carcinoma: mammographic findings in a 10-year experience. Radiology. 1991; 178:149–154.

7. Skaane P, Skjørten F. Ultrasonographic evaluation of invasive lobular carcinoma. Acta Radiol. 1999; 40:369–375.

8. Watermann DO, Tempfer C, Hefler LA, Parat C, Stickeler E. Ultrasound morphology of invasive lobular breast cancer is different compared with other types of breast cancer. Ultrasound Med Biol. 2005; 31:167–174.

9. Rodenko GN, Harms SE, Pruneda JM, Farrell RS Jr, Evans WP, Copit DS, et al. MR imaging in the management before surgery of lobular carcinoma of the breast: correlation with pathology. AJR Am J Roentgenol. 1996; 167:1415–1419.

10. Kim SH, Cha ES, Park CS, Kang BJ, Whang IY, Lee AW, et al. Imaging features of invasive lobular carcinoma: comparison with invasive ductal carcinoma. Jpn J Radiol. 2011; 29:475–482.

11. Liberman L, Morris EA, Kim CM, Kaplan JB, Abramson AF, Menell JH, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003; 180:333–341.

12. Mameri CS, Kemp C, Goldman SM, Sobral LA, Ajzen S. Impact of breast MRI on surgical treatment, axillary approach, and systemic therapy for breast cancer. Breast J. 2008; 14:236–244.

13. Lau B, Romero LM. Does preoperative magnetic resonance imaging beneficially alter surgical management of invasive lobular carcinoma. Am Surg. 2011; 77:1368–1371.

14. Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, et al. The effects of preoperative (18)F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer. 2012; 15:441–448.

15. Groves AM, Shastry M, Ben-Haim S, Kayani I, Malhotra A, Davidson T, et al. Defining the role of PET-CT in staging early breast cancer. Oncologist. 2012; 17:613–619.

16. Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000; 18:3495–3502.

17. Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004; 31:Suppl 1. S80–S87.

18. American College of Radiology. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 4th ed. Reston: American College of Radiology;2003.
19. Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2003; 180:901–910.

20. Liberman L, Morris EA, Lee MJ, Kaplan JB, LaTrenta LR, Menell JH, et al. Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol. 2002; 179:171–178.

21. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004; 6:R149–R156.

22. Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002; 20:379–387.

23. Vandorpe T, Smeets A, Van Calster B, Van Hoorde K, Leunen K, Amant F, et al. Lobular and non-lobular breast cancers differ regarding axillary lymph node metastasis: a cross-sectional study on 4,292 consecutive patients. Breast Cancer Res Treat. 2011; 128:429–435.

24. Kneeshaw PJ, Turnbull LW, Smith A, Drew PJ. Dynamic contrast enhanced magnetic resonance imaging aids the surgical management of invasive lobular breast cancer. Eur J Surg Oncol. 2003; 29:32–37.

25. McGhan LJ, Wasif N, Gray RJ, Giurescu ME, Pizzitola VJ, Lorans R, et al. Use of preoperative magnetic resonance imaging for invasive lobular cancer: good, better, but maybe not the best? Ann Surg Oncol. 2010; 17:Suppl 3. 255–262.

26. Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol. 2004; 183:1149–1157.

27. Mann RM, Loo CE, Wobbes T, Bult P, Barentsz JO, Gilhuijs KG, et al. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2010; 119:415–422.

28. Pediconi F, Catalano C, Roselli A, Padula S, Altomari F, Moriconi E, et al. Contrast-enhanced MR mammography for evaluation of the contralateral breast in patients with diagnosed unilateral breast cancer or high-risk lesions. Radiology. 2007; 243:670–680.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download