Abstract
Phyllodes tumors are an infrequent breast tumor presentation. A phyllodes tumor with a synchronous invasive ductal carcinoma is rarely described and has never been reported with lobular carcinoma in situ component. A 53-year-old female presented with a nine-year history of twice core biopsy proven fibroadenoma. After an increase in the tumor's growth velocity it was decided upon to undergo an excisional biopsy. Microscopic examination of the well-circumscribed pale-tan mass found focal areas of leaf like architecture with variable number of mitoses present, representing a phyllodes tumor of borderline malignant potential. Incidentally, at one edge of the mass was found a tubular carcinoma and lobular carcinoma in situ components. Thorough, routine follow-up of patients with biopsy proven benign breast masses is important to finding a masked malignant component.
Phyllodes tumor is a rare fibroepithelial tumor of the breast that accounts for less than 1% of all breast tumors [1]. Few cases have been published involving a synchronous invasive carcinoma component within a phyllodes tumor [2-7]. This is the first documented case of a borderline malignant phyllodes tumor with both tubular carcinoma and lobular carcinoma in situ components within the same tumor mass.
A 53-year-old female presented with a several year history of a right painless breast mass in the right upper outer quadrant that had grown in size significantly during the last two years until referral for treatment. The patient had no increased risk factors and family history was negative for both breast and ovarian cancers. The patient had been started on progesterone 10 mg daily one year prior to presentation for oligomenorrhea. A core biopsy taken nine years prior to presentation was reported as fibroadenoma. The patient was then followed biennially by the Ontario Breast Screening Program until the significant growth increase when a second core biopsy was recommended and was also consistent with fibroadenoma. Given the sudden increase in size, with the lesion now measuring 5.5×1.8 cm by mammogram (Figures 1, 2), the radiological/pathological correlation rounds recommended a surgical assessment and excisional biopsy for definitive diagnosis. An excisional biopsy was undertaken.
Grossly, the well-circumscribed pale-tan mass measured 6.5×3.6×2.4 cm, which in areas had a whorled appearance. Histologically, focal areas of fibroadenomatous changes were noted (Figure 3A) while areas of leaf like architecture with variable cellularity of stromal component were present as well. Cytologic atypia was present throughout the stromal component ranging from minimal to moderate atypia and a variable number of mitoses were present (up to 7 to 8 per 10 high power fields), consistent with a diagnosis of phyllodes tumor with borderline malignant potential (Figure 3B). At the lateral portion of the excised mass there was a tubular carcinoma area with a greatest dimension of 2.4 cm (Figure 3C). The carcinoma cells were immunohistochemically positive for epithelial membrane antigen, estrogen receptor, progesterone receptor and E-cadherin, but negative for S100 protein. Absence of basal myoepithelial lining in tubules was confirmed by negativity for smooth muscle myosin heavy chain and P63 (Figure 3D). Lobular carcinoma in situ was also noted in this area and the diagnosis was confirmed by negativity of immunohistochemical stain for E-cadherin (Figure 3E, 3F). Microcalcification was observed within the tubular carcinoma. The surgical margins were not involved by the tumor, but the tubular carcinoma was very close (0.1 mm) to the inked surface at the lateral and posterior edges.
Following the pathology review the patient had complete staging workup, including bone scan, liver ultrasound, and chest X-ray. These were all negative for metastases. A dedicated breast magnetic resonance image (MRI) was completed prior to reexcision that demonstrated a few, scattered enhancing masses of unknown significance. Subsequently the patient underwent a re-excision for margin safety and sentinel lymph node biopsy. Pathology review of the re-excision found scerlosing adenosis and atypical ductal hyperplasia with no evidence of residual invasive or in situ carcinoma or phyllodes tumor components. The previously close margins of tubular carcinoma at the lateral and posterior aspect are now 4.0 cm and 2.8 cm from those margins, respectively. All three sentinel nodes were negative for malignancy.
This patient's unique presentation was reviewed at breast tumor rounds at the Juravinski Cancer Centre with the decision for the patient to next be evaluated by the radiation oncology team for adjuvant therapy.
Phyllodes tumors are rare breast fibroepithelial neoplasms that can be potentially aggressive. They are usually classified as benign, borderline, or malignant, depending upon stromal cellularity and overgrowth, cellular atypia, mitotic activity, and margin status. The local recurrence for these tumors range from 4.3-36% with wide local excision (>1 cm margin), the standard primary treatment modality [8,9]. Additionally, distant metastasis can occur in 10% of these patients. An invasive ductal carcinoma that is incidentally found within a borderline phyllodes tumor has been reported only once before in literature [10]. Our case showed a similar presentation to the previous one, and a lobular carcinoma in situ component was also accompanied with the phyllodes tumor and invasive tubular carcinoma.
This patient was followed routinely for several years with a stable lesion, originally biopsied as fibroadenoma. During the interim the mass followed what is expected as a normal course for a fibroadenoma: overall stable size, sometimes minimal size alteration with menstrual cycles, painless, and well-circumscribed on radiographs. Given that both fibroadenomas and phyllodes tumors have similar clinical presentation of a painless breast mass, physicians rely on accurate diagnostic imaging, biopsy, and routine clinical follow-up for surveillance. A retrospective review of thirty-one histologically proven masses (19 fibroadenomas and 12 phyllodes tumors) examined the mammographic and sonographic differences between fibroepithelial lesions [11]. From that review the authors concluded there is substantial overlap in the radiographic characteristics of these tumors. They suggested that assured accurate diagnosis can only be made by core or excisional biopsy.
As a less invasive diagnostic modality to excisional biopsy, core biopsy of fibroepithelial lesions is a strong alternative for differentiating these tumors. In a study of sixty-seven Finnish females examining the efficacy of core biopsy of diagnosing between fibroadenoma and phyllodes tumor, they found a sensitivity, specificity, and positive and negative predictive values of 83%, 92%, 71%, and 96%, respectively, for phyllodes tumors [12]. In this patient, even though the most recent repeat needle core biopsy confirmed again a fibroadenoma it was the significant change in size of the tumor that raised concern of a more aggressive mass, leading to excision and incidentally finding the invasive carcinoma component. Unfortunately, review of this patient's mammograms did not suggest an invasive carcinoma component, given that it was within the well-circumscribed borderline phyllodes component.
In conclusion, a phyllodes tumor with a synchronous invasive carcinoma component is rarely presented and routine follow-up that involves clinical and radiologic assessment of fibroadenomas is important to curtail progression of a masked malignant component.
Figures and Tables
Figure 1
Ultrasound (US) of right breast multilobulated, solid mass measured 4.91×1.76 cm. Mildly heterogenous, paralleling the skin surface. In 2001 the tumor's longest US dimension was 2.4 cm.
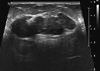
Figure 2
Mammography of right breast, craniocaudal view, showed a circumscribed, multilobulated solid mass.
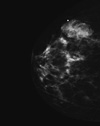
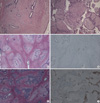
Figure 3
(A) The mass revealed fibroadenomatous change (H&E stain, ×40). (B) Leaf-like structures of fibroepithelial lesion showed a cellular stroma with cytological atypia and increased mitosis (H&E stain, ×40). (C) Invasive carcinoma cells formed small tubules within the stroma (H&E stain, ×20). (D) Tubules of carcinoma cells were immunohistochemically negative for myoepithelial marker, p63 (IHC, ×40). (E) Lobular carcinoma in situ was noted within the glandular component (H&E stain, ×40). (F) Lobular carcinoma in situ was immunohistochemically negative for E-cadherin (×40).
References
1. Rosen PP, Oberman HA. Rosai J, Sobin LH, editors. Cystosarcoma phyllodes. Atlas of Tumor Pathology. Tumors of the Mammary Gland. 1993. 3rd series. Washington, DC: Armed Forces Institute of Pathology;101–114.
2. Ozzello L, Gump FE. The management of patients with carcinomas in fibroadenomatous tumors of the breast. Surg Gynecol Obstet. 1985. 160:99–104.
3. Parfitt JR, Armstrong C, O'malley F, Ross J, Tuck AB. In-situ and invasive carcinoma within a phyllodes tumor associated with lymph node metastases. World J Surg Oncol. 2004. 2:46.
4. Kodama T, Kameyama K, Mukai M, Sugiura H, Ikeda T, Okada Y. Invasive lobular carcinoma arising in phyllodes tumor of the breast. Virchows Arch. 2003. 442:614–616.

5. Macher-Goeppinger S, Marme F, Goeppert B, Penzel R, Schirmacher P, Sinn HP, et al. Invasive ductal breast cancer within a malignant phyllodes tumor: case report and assessment of clonality. Hum Pathol. 2010. 41:293–296.

6. Sugie T, Takeuchi E, Kunishima F, Yotsumoto F, Kono Y. A case of ductal carcinoma with squamous differentiation in malignant phyllodes tumor. Breast Cancer. 2007. 14:327–332.

7. de Rosa G, Ferrara G, Goglia P, Ghicas C, Zeppa P. In situ and microinvasive carcinoma with squamoid differentiation arising in a phyllodes tumor: report of a case. Tumori. 1989. 75:514–517.

8. Reinfuss M, Mituś J, Duda K, Stelmach A, Ryś J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996. 77:910–916.

9. Barth RJ Jr. Histologic features predict local recurrence after breast conserving therapy of phyllodes tumors. Breast Cancer Res Treat. 1999. 57:291–295.

10. Christensen L, Nielsen M, Madsen PM. Cystosarcoma phyllodes. A review of 19 cases with emphasis on the occurrence of associated breast carcinoma. Acta Pathol Microbiol Immunol Scand A. 1986. 94:35–41.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download