Abstract
Purpose
Primary systemic therapy (PST) downstages up to 40% of initial documented axillary lymph node (ALN) metastases in breast cancer. The current surgical treatment after PST consists of breast tumor resection and axillary lymph node dissection (ALND). This strategy, however, does not eliminate unnecessary ALND in patients with complete remission of axillary metastases. The aim of this study was to examine the accuracy of sentinel lymph node biopsy (SLNB) after PST among patients with documented ALN metastasis at presentation and to identify the rate of pathologic complete-remission (CR) with ALN after PST.
Methods
We analyzed 66 patients with ALN metastasis that was pathologically proven preoperatively who underwent SLNB and concomitant ALND after PST. Axillary ultrasound (AUS) was used to evaluate the clinical response of initially documented ALN metastasis after PST. Intraoperative lymphatic mapping was performed using blue dye with or without radioisotope.
Results
After PST, 34.8% of patients had clinical CR of ALN on AUS and 28.8% patients had pathologic CR of ALN. The overall success rate of SLNB after PST was 87.9%, and the sentinel lymph node identification rate in patients with clinical CR was 95.7%. In patients with successful lymphatic mapping, 70.7% of patients had residual axillary metastases. The overall accuracy and false-negative rate were 87.9% and 17.1% in all patients: 95.5% and 10.0% in patients with clinical CR of ALN, and 83.3% and 19.4% in patients with residual axillary disease after PST.
Primary systemic therapy (PST) for breast cancer was initially used in cases of locally advanced inoperable disease in order to achieve surgical resection [1]. The use of PST was extended to operable breast cancer regardless of axillary status based on results showing that disease-free and overall survival obtained with adjuvant chemotherapy improved and more patients were able to receive breast-conserving surgery [2,3].
Sentinel lymph node biopsy (SLNB) has been accepted as an accurate and safe alternative to axillary lymph node dissection (ALND) for early breast cancer, and is associated with negligible morbidity compared with ALND [4-6]. However, SLNB is not recommended for axillary staging after PST because of low detection rates in several retrospective, single-institution studies [7,8]. However, a recent meta-analysis and prospective multicenter study suggested the feasibility of SLNB after PST by showing that the detection rate and false-negative (FN) rate were similar to those reported for early-stage breast cancer without PST [9,10]. The results of the National Surgical Adjuvant Breast and Bowel Project B27 have also shown that there are no significant differences in the detection rate and FN rate of SLNB after PST according to clinical nodal status [11].
PST has been shown to downstage up to 40% of pre-PST documented axillary lymph node (ALN) metastases and to eradicate biopsy-proven ALN metastases in 32% of patients [12-14]. Although axillary downstaging has been observed after PST, standard surgical treatment after PST consists of primary breast tumor resection and level I-II ALND. SLNB is performed prior to PST to achieve more accurate nodal staging. This strategy may neglect the fact that patients with complete tumor response in metastatic axillary lymph nodes could be spared from undergoing ALND. Therefore, it is important to ascertain whether SLNB after PST can accurately identify patients for whom ALND can be avoided.
The objective of this study was two-fold. First, we examined the accuracy of SLNB after PST among patients with breast cancer who had pathologically proven ALD metastases at the initial diagnosis. Second, we identified the rates of clinical and pathologic complete remission (CR) in the ALN after PST. In addition, we compared the differences in SLNB accuracy according to clinically axillary response after PST.
From August 2003 to June 2009, 169 patients with breast cancer who underwent surgical treatment after PST at Seoul National University Bundang Hospital were retrospectively identified from the hospital's surgical database. All patients were free of distant metastases and had T1 through T4, N0 through N3 breast cancer at the time of initial diagnosis. SLNB and concomitant ALND were performed prospectively for 89 patients after PST. Among these patients, fine-needle aspiration cytology (FNAC) was performed in 70 patients with clinically suspicious ALNs on ultrasonographic findings. Sixty-six patients presented with pathologically proven ALN metastases, and these patients constituted the present study sample. This retrospective study was approved by our Institutional Review Board.
At our institute, patients undergo axillary ultrasound (AUS) for initial axillary staging at presentation as well as clinical response in axilla after PST. Initial AUS findings and follow-up AUS imaging after PST were reviewed by a single radiologist with specific expertise in breast imaging at our institution. Suspicion of AUS was based on the presence of an asymmetric, hypoechoic prominence of the cortex or loss of fatty hilum. FNAC of suspicious lymph nodes was performed by a radiologist using ultrasound guidance to target the abnormal areas of the nodes. All patients diagnosed with axillary metastases underwent AUS to evaluate the clinical response in breast and ALNs after PST. We divided patients into two groups according to clinical axillary response using ultrasound findings as follows: normal and fatty replaced lymph nodes on axillary ultrasound were classified as clinical CR, and suspicion of residual disease was classified as clinical non-CR. Dissected ALNs were evaluated for pathologic tumor response in axillary metastases. Patients who had no metastatic carcinoma in the dissected nodes were considered to have had a pathologic CR for metastatic ALNs. Pathologic CR for primary breast tumor was considered to indicate no invasive cancer. Estrogen and progesterone receptors were scored as percentage of cells which stain with the antibody. Until May 2010, the cancer was classified as hormone receptor positive in our institution if there were at least 10% positive tumor nuclei in the sample on testing in the presence of expected reactivity of internal (normal epithelial elements) and external controls. Comparisons of post-PST AUS findings and final pathology of ALNs were performed to assess whether ultrasound could predict complete tumor response in the axilla.
Intraoperative lymphatic mapping was performed using blue dye with or without radioisotope. Periareolar injection of 99mTc-labeled antimony sulfur colloid (Seoul National University Bundang Hospital, Seongnam, Korea) was performed 1-3 hours before the operation, and 5 mL of 0.8% indigocarmine (Korea United Pharm Inc., Seoul, Korea) was injected intraoperatively. All sentinel lymph nodes (SLNs) were examined intraoperatively by serial sectioning and hematoxylin and eosin staining. Additional immunohistochemical labeling for cytokeratin was performed to further evaluate the status of SLNs on permanent section. All patients underwent concomitant ALND after SLNB.
The success rate of SLNB was defined as the number of patients whose SLN was isolated in relation to the total number of patients in this study. FN rate was defined as the ratio of the number of patients with a FN case of SLNB to the number of patients with at least one involved SLN or non-SLN. Comparisons of the success and FN rates according to clinical response after PST were performed using Fisher's exact tests, and p<0.05 was considered statistically significant.
Table 1 shows the clinicopathologic characteristics of the 66 patients. The average clinical tumor size of 45 patients (68.2%) was between 2 cm and 5 cm, and 13 patients (19.7%) had a large tumor (>5 cm) at the time of diagnosis. Prior to surgery, 35 patients (53.0%) were treated with four cycles of a combination of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), and 28 patients (42.4%) received three or six cycles of a combination of docetaxel (75 mg/m2) and doxorubicin (50 mg/m2). One patient was treated with three cycles of a combination of docetaxel (75 mg/m2) and epirubicin (100 mg/m2), and two patients with six cycles of a combination of paclitaxel (175 mg/m2), gemcitabine (1,200 mg/m2), and trastuzumab (4 mg/kg). Twenty-eight patients (42.4%) underwent a total mastectomy for their primary tumors, and 38 patients (57.6%) underwent breast conserving surgery. Fourteen patients (21.2%) had complete pathologic response in the primary breast tumor after PST.
On initial AUS findings, the most common characteristics of abnormal lymph nodes were uneven cortical thickening (47.0%) and round hypoechoic mass (45.5%). Enlarged lymph nodes were spread throughout level I only (71.0%), level I and II (25.8%), and level I, II and III (1.5%). The mean number of enlarged lymph nodes was 3.0 (range, 1-10). After PST, 43 patients had clinically residual nodal disease and 23 patients had a clinical CR in axilla on ultrasound examination. Nineteen (28.8%) of the 66 patients with metastatic ALN showed pathologic CR of axillary metastases at the time of SLNB and completion of ALND. Six (14.0%) of 43 patients with clinical non-CR and 13 (52.2%) of 23 patients with clinical CR were found to have pathologic CR on final pathology. The AUS for evaluating the tumor response in metastatic ALNs after PST had a sensitivity of 78.7%, a specificity of 68.4%, a positive predictive value of 86.0%, a negative predictive value of 52.2%, and an accuracy of 75.8% (Table 2).
Lymphatic mapping was performed with both radioactive colloid and blue dye in 61 patients, and with blue dye alone in five patients. The mean number of SLNs removed was 2.8 (range, 1-8), and the SLNs were located in the axilla in all patients. The mean number of nodes retrieved after completion of ALND was 19.9 (range, 5-44). The overall SLN identification rate after PST was 87.9% (58/66). According to clinical tumor response in the axilla, the identification rates of SLN between clinical CR and non-CR were 95.7% (22/23) and 83.7% (36/43), however, the difference was not statistically significant (p=0.244). In 58 patients who had successful lymphatic mapping, 41 patients (70.7%) had pathologically residual axillary metastases. The pathologic status of the SLN and ALN in all patients is showed in Table 3. The SLN accurately predicted axillary nodal status in 51 of 58 patients (overall accuracy, 87.9%). Seven patients had FN findings, the overall FN rate of 17.1% (7/41).
In the SLNB success group, 22 patients had clinical CR in the axilla, and 36 patients had abnormal appearance of the ALNs. Among patients with clinical non-CR, the SLN accurately predicted axillary nodal status in 30 of 36 patients (83.3%) and the FN rate was 19.4% (Table 4). In patients with clinical CR, the overall accuracy and FN rate were 95.5% and 10.0% (Table 5). However, there were no significant differences in FN rates according to clinical tumor response in axilla (p=0.660). There were no significant differences in the success rates for SLNB and FN rate according to age, clinical and pathologic tumor size, type of PST, or hormonal receptor (data not shown).
In early breast cancer with clinically negative ALN, SLNB is an accurate predictor of the pathologic status of ALNs. However, some have speculated that the disease status of the SLN in patients who receive PST does not accurately reflect the disease status of entire axilla due to fibrosis of tumor lymphatics and the potential obstruction of lymphatics by cellular material or tumor emboli [15,16]. Therefore, it has been suggested that SLNB should be performed before PST. There are some disadvantages to this approach, because patients would have at least two operations and patients with nodal disease and evidence of nodal regression would not have the chance to avoid ALND, which is associated with morbidity.
There have been many studies evaluating the feasibility of SLNB after PST, and identification rates of 72% to 100% and false negative rates of 0% to 33% have been reported [7,17-19]. According to meta-analyses of these studies, the estimated identification rate for SLNB after PST was 91% and the sensitivity of SLNB was 88%, with a FN rate of 12% [9]. These results suggest that SLNB is a reliable technique for determining the need for ALND in clinically node-negative patients after PST. Previously, several studies of the feasibility of SLNB after PST in patients with cytologically documented positive lymph nodes at presentation were conducted. Among 793 patients from 19 studies, the identification of SLN was 85%, in half of studies it was 90% or higher, and the FN rate was 11% [20]. These findings suggest that SLNB after PST is reasonable in patients with positive node.
In our study, the post-PST SLNB in patients with axillary metastases at presentation did not seem to be feasible, as there was a higher false negative rate of 17.1% than previous results from conventional lymphatic mapping in early breast cancer. Although there were no statistically significant differences between patients with clinically CR and patients with residual adenopathy, we observed lower FN rates and higher identification rates of SLNB in patients with clinical CR (10%, 95.7%) than in patients with clinical non-CR (19.4%, 83.7%) for nodal disease after PST. These findings suggest that SLNB may be feasible in patients with imaging evidence of CR for their axillary disease after PST.
PST has been shown to sterilize cytologically proven metastatic ALNs in 23% to 32% of patients [12,13]. In our study, 28.8% of patients with axillary metastases at diagnosis had a pathologically complete response, and ALND might not be necessary in such patients. Metastatic lymph node response to chemotherapy is also important to provide prognostic information. Kuerer et al. [12] showed that complete remission of nodal metastases after PST was a strong predictor of disease-free survival (87% in patients with preoperative eradication of axillary metastases vs. 51% in patients with residual nodal disease after 5 years), and also that the 5-year disease-free survival rate in patients with occult nodal metastasis was not significantly different from that in patients without occult nodal metastasis (87% vs. 75%, respectively, after 5 years). Based on the results of The National Surgical Adjuvant Breast and Bowel Project B-04 trial, although 40% of breast cancer patients with clinically negative nodes have pathologically positive nodes, no survival advantage is associated with removing occult positive nodes at the time of initial surgery [21]. These studies support the use of SLNB as an alternative to ALND after PST.
Ultrasound is the most easily-applicable imaging tool for clinical staging of ALN status in patients with breast cancer [22]. AUS, possibly in combination with FNAC of suspicious nodes has been identified as a preoperative procedure for the selection of breast cancer patient suitable for SLNB [23,24]. Our diagnoses were determined by AUS examination and ultrasound-guided FNAC of suspicious nodes. Therefore, we selected AUS as the follow-up modality for comparison. However, our ability to identify patients with clinically negative axilla after PST was suboptimal. Results of the present study suggest that AUS is not reliable enough to predict pathologic status of the axilla after PST, with a low specificity of 68.4% and a low negative predictive value 52.2%. Therefore, another imaging modality with better sensitivity and specificity such as positron emission tomography (PET) should be combined with AUS to identify patients who have clinically negative axilla after PST and who are appropriate candidates for SLNB. Recently, Straver et al. [25] demonstrated that monitoring the response of ALNs with FDG PET/CT during the early course of preoperative chemotherapy is feasible, with a reported sensitivity of 97% and specificity of 100%.
The small sample size and single institution survey are limitations of our study. However, most of the SLNB were performed using a combination of blue dye and radioisotope, and the surgeon analyzed all SLNs in the setting of planned completion of ALND.
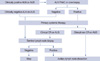
In summary, our study was designed to evaluate the feasibility and accuracy of SLNB after PST in patients with axillary metastases at diagnosis. Although the overall FN rate was higher than that of early breast cancer, the FN rate in patients with clinical CR of nodal disease to PST was 10%. It suggests that SLNB may be feasible in patients with clinically complete axillary response following PST. If this result is confirmed in a large randomized trial, SLNB will be generally accepted as the standard method for axillary staging in this group. We suggest a new algorithm of surgical treatment for axillary staging after PST (Figure 1), and expect that some patients with clinical CR of nodal metastases may be able to avoid unnecessary ALND.
Figures and Tables
Figure 1
New suggested algorithm to prevent unnecessary axillary lymph node dissection after primary systemic therapy.
ALN=axillary lymph node; FNAC=fine needle aspiration cytology; AUS=axillary ultrasound; CR=complete remission.
References
1. Bonadonna G. Evolving concepts in the systemic adjuvant treatment of breast cancer. Cancer Res. 1992. 52:2127–2137.
2. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998. 16:2672–2685.

3. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001. 19:4224–4237.

4. Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006. 106:4–16.

5. Naik AM, Fey J, Gemignani M, Heerdt A, Montgomery L, Petrek J, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004. 240:462–468.
6. Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000. 88:608–614.

7. Nason KS, Anderson BO, Byrd DR, Dunnwald LK, Eary JF, Mankoff DA, et al. Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer. 2000. 89:2187–2194.

8. Fernández A, Cortés M, Benito E, Azpeitia D, Prieto L, Moreno A, et al. Gamma probe sentinel node localization and biopsy in breast cancer patients treated with a neoadjuvant chemotherapy scheme. Nucl Med Commun. 2001. 22:361–366.

9. Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006. 93:539–546.

10. Classe JM, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009. 27:726–732.

11. Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005. 23:2694–2702.

12. Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999. 230:72–78.

13. Newman LA, Pernick NL, Adsay V, Carolin KA, Philip PA, Sipierski S, et al. Histopathologic evidence of tumor regression in the axillary lymph nodes of patients treated with preoperative chemotherapy correlates with breast cancer outcome. Ann Surg Oncol. 2003. 10:734–739.

14. Newman EA, Sabel MS, Nees AV, Schott A, Diehl KM, Cimmino VM, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007. 14:2946–2952.

15. Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol. 1996. 9:893–900.
16. Kuerer HM, Hunt KK. The rationale for integration of lymphatic mapping and sentinel node biopsy in the management of breast cancer after neoadjuvant chemotherapy. Semin Breast Dis. 2002. 5:80–87.
17. Schwartz GF, Meltzer AJ. Accuracy of axillary sentinel lymph node biopsy following neoadjuvant (induction) chemotherapy for carcinoma of the breast. Breast J. 2003. 9:374–379.

18. Patel NA, Piper G, Patel JA, Malay MB, Julian TB. Accurate axillary nodal staging can be achieved after neoadjuvant therapy for locally advanced breast cancer. Am Surg. 2004. 70:696–699.
19. Kang SH, Kim SK, Kwon Y, Kang HS, Kang JH, Ro J, et al. Decreased identification rate of sentinel lymph node after neoadjuvant chemotherapy. World J Surg. 2004. 28:1019–1024.

20. Dixon JM, Cody HS 3rd. Role of sentinel node biopsy in patients having neoadjuvant chemotherapy. Eur J Surg Oncol. 2010. 36:511–513.

21. Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002. 347:567–575.

22. Nori J, Vanzi E, Bazzocchi M, Bufalini FN, Distante V, Branconi F, et al. Role of axillary ultrasound examination in the selection of breast cancer patients for sentinel node biopsy. Am J Surg. 2007. 193:16–20.

23. Sato K, Tamaki K, Tsuda H, Kosuda S, Kusano S, Hiraide H, et al. Utility of axillary ultrasound examination to select breast cancer patients suited for optimal sentinel node biopsy. Am J Surg. 2004. 187:679–683.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download