Abstract
Recently remarkable progress has been made in the understanding of lymphangiogenesis due to the availability of specific biomarkers. A sentinel lymph node (SLN) biopsy has already been established as a common surgical procedure, and the clinical usefulness of an SLN biopsy has been confirmed in patients with various types of cancer. In this study, a novel method has been successfully developed for the isolation of anatomically defined lymphatic endothelial cells (LECs) from human sentinel lymphatic channels during an SLN biopsy in breast cancer patients by the use of collagenase II digestion. The isolated cells were cultured in EGM-2MV media with 10% fetal bovine serum (FBS) under hypoxic conditions in an atmosphere of 5% O2, 5% CO2 and 90% N2 at 37℃. The cultured cells exhibited a monolayer with a cobblestone appearance. Immunofluorescence analysis using selective lymphangiogenesis markers showed expression of vascular endothelial growth factor receptor 3 (VEGFR-3), prospero homeobox 1 (Prox-1) and Podoplanin. Newly established LECs showed expressions of vascular endothelial growth factor (VEGF) family members except VEGF-D and corresponding VEGF receptors by the use of conventional RT-PCR. Treatment with VEGF-C156S (500 ng/mL) apparently induced phosphorylation of the protein kinase Akt, MAPK and JNK in human isolated LECs as determined by Western blot analysis. Peak induction of Akt, MAPK and JNK occurred at 10 to 15 minutes after incubation of the isolated LECs with VEGF-C156S. The use of the MTS cell proliferation assay showed a significant increase in the growth of human lymphatic endothelial cells with VEGF-C156S treatment. The effects of VEGF-C156S (500 ng/mL) on proliferation activity was significantly with 3% FBS condition alone (MTS score: 0.54±0.0104, n=3) and a 3% FBS+VEGF-C156S (MTS score: 0.572±0.0061, n=3) condition. The isolated and enzymatic digested method adopted for the culture of human LECs is simple and useful for the investigation of the cellular, molecular and genomic properties of LECs.
A lymph node is an organ consisting of many types of cells, and is a part of the lymphatic system. Lymph nodes are found throughout the body, and lymph nodes act as filters or traps for foreign particles and contain white blood cells. The condition of lymph nodes is so significant that the condition of the nodes is used for cancer staging, to decide the treatment modalities to be employed, and to determine prognosis. A sentinel lymph node (SLN) biopsy has already been established as a common surgical procedure, and its clinical usefulness has been confirmed in patients with various types of cancer.(1-3) The lymph node that tumor cells reach first through the lymphatic vessels is the "sentinel lymph node", and the lymphatic endothelial cells that comprise the existing lymphatic vessels are called sentinel lymphatic endothelial cells. Recently, based on the discovery of vascular endothelial growth factor (VEGF)-C and VEGF-D, which are growth factors directly controlling lymphangiogenesis, and VEGFR-3, a tyrosine kinase receptor involving these factors, it has been reported that metastasis to regional lymph nodes occurs through peritumoral lymphangiogenesis in experimental animal models.(4) Thus the relationship between lymphangiogenesis and metastasis to the lymph nodes has been demonstrated.(5) The lymph fluid circulates to the lymph node via afferent lymphatic vessels and drains into the lymph node, which acts to trap foreign particles and filter the lymph fluid. The lymph fluid then exits the lymph node via the efferent lymphatic vessel towards either a more central lymph node or ultimately for drainage into a central venous subclavian blood vessel. It has been considered that newly generated lymphatic vessels induced during the growth process of cancer tissue, form communications with existing lymphatic vessels, and that cancer cells flow along these communication pathways into the lymphatic vessels, resulting in arrival at the sentinel lymph node. Sentinel lymphatic endothelial cells are always exposed to various types of materials produced by cancer cells. No reports can be found on the development of sentinel lymphatic endothelial cells from lymphatic vessels collected at the time of an SLN biopsy. A novel method has been successfully developed for the isolation of anatomically defined lymphatic channels and for the establishment of the sentinel lymphatic endothelial cells in vitro. In this study, the characterization of isolated sentinel lymphatic endothelial cells in breast cancer patients that have undergone a sentinel lymphadenectomy is described.(6)
This study was undertaken using an institutional review board approved protocol of Saitama Medical University Hospital. A stereomicroscopic image of lymph nodes and lymphatic vessels collected at the time of an SLN biopsy is shown in Figure 1. Perioperative identification of dye-stained lymphatic vessels (Figure 1A) and extraction of the lymphatic vessels were performed by dissecting connective and adipose tissues around the afferent lymph vessels using a dissecting microscope. Micro-clips were placed at both ends of the vessels to prevent dye leakage (Figure 1B). Short segments of lymphatic channels were obtained without the surrounding tissues, which were cannulated under the stereomicroscope using a sterile polyethylene silicon tube with a diameter of less than 0.5 mm. The tube and vessel were fixed with pieces of string (Figure 1C). Following perfusion with phosphate-buffered saline (PBS) solution to remove intraluminal fluid, the lumen of the vessels was slowly circulated through the tube with 0.05% collagenase II solution (Worthington, Lakewood, USA) for approximately 10 min. The collected solution was centrifuged at 2,000 rpm for 5 min at 4℃. The supernatant was discarded and the pellets were resuspended in enriched growth factor media, EGM-2MV (Clonetics, San Diego, USA) containing 10% fetal bovine serum (FBS). Collected lymphatic endothelial cells were plated onto a 6-well culture plate coated with gelatin (BD Biosciences, San Jose, USA). All lymphatic endothelial cells were incubated under an atmospheric condition of 5% O2, 5% CO2, and 90% N2 at 37℃. Cells were subcloned when confluence was reached after treatment with trypsin/ EDTA and cells were used at the fourth to sixth passage for the study.(7-9)
Collected cells using the collagenase II perfusion solution were transferred to the culture system, and colonies considered as endothelial cells morphologically were confirmed (Figure 2). The cultured cells exhibited a monolayer with a cobblestone appearance. To determine further that the cells were derived from lymphatic vessels and were transferred into the culture system, immunofluorescence analysis was performed for the isolated lymphatic endothelial cells. The cells were seeded on glass slides coated with gelatin and cells were grown to 80% confluence. The cells were then fixed with 10% paraformaldehyde neutral buffer solution followed by blocking in 1% bovine serum albumin. Cells were incubated with primary antibodies overnight at 4℃ and then with secondary antibodies such as FITC-conjugated anti-rabbit antibodies (1:200, Biotechnology, Santa Cruz, USA) for 30 min at room temperature. Pretreatment with 0.1% Triton-X (Sigma, St Louis, USA) for five minutes was needed for the use of the anti-Prox-1 antibody that could interact with antigen in the nucleus. The following primary antibodies were used: anti-Prox-1 (1:50 dilution, AngioBio, Del Mar, USA), anti-podoplanin (1:50 dilution, AngioBio, Del Mar, USA) and anti-VEGFR3 (1:50 dilution, R&D Systems, Minneapolis, USA) when compared to DAPI counterstaining. These antibodies were directed against selective markers of lymphatic endothelial cells. After washing with PBS, cells were mounted in MobiGLOW Mounting Medium (Mo Bi Tec, Göttingen, Germany) and were viewed with the use of under fluorescence microscopy. All images were recorded by the use of a video camera and images were converted to JPEG files. Merged images of immunofluorescence multicolor staining were made using Adobe Photoshop Software (Adobe Systems, San Jones, USA). The immunoreactions of all markers were intense for almost cultured cells, which were localized as seen on the cytoplasmic field (VEGFR-3 and podoplanin) or in the nuclei (Prox-1) (Figure 2).
RNA expression of VEGF family proteins and corresponding receptors was first evaluated by the use of RT-PCR. Total RNA was extracted from each sample using TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, USA). First strand cDNA was synthesized from 5 µg of total RNA using SuperScript III RNase H- reverse transcriptase (Invitrogen, Carlsbad, USA) in a total reaction volume of 20 µL containing 0.5 mM dNTPs mixture and 0.025 µg/µL oligo(dT)12-18 primer. PCR analysis was performed using a pair of forward and reverse primers for the VEGF family members and receptors. The primer sequences of all genes that were used are shown in Figure 3. The PCR reactions contained 1 µL cDNA, 1×PCR buffer (Takara, Shiga, Japan), 200 mM dNTPs each, 0.5 units of Ex Taq Polymerase (Takara, Shiga, Japan), and 0.2 µM each of the primers. The PCR reactions were subjected to the following amplification scheme. For VEGFR-3, 1 cycle of 94℃ for 2 min, 35 cycles of 94℃ for 1 min, 56℃ for 1 min, and 72℃ for 2 min, and a final extension step at 72℃ for 5 min after the last cycle. For the other genes, 1 cycle of 95℃ for 2 min, 30-35 cycles of 95℃ for 30 sec, 56℃ (VEGF-C, VEGF-D and VEGFR-2) or 60℃ (VEGF-A, VEGF-B, VEGFR-1, NRP-1 and NRP-2) for 30 sec, and 72℃ for 1 min, and a final extension step at 72℃ for 5 min after the last cycle. For GAPDH, 1 cycle of 95℃ for 2 min, 25 cycles of 95℃ for 30 sec, 56℃ for 30 sec, and 72℃ for 1 min, and a final extension step at 72℃ for 5 min after the last cycle. Amplified fragments were visualized by ethidium bromide staining of a 2% agarose gel and gels were photographed under UV light. RT-PCR showed expression of VEGF-A, VEGF-B, VEGF-C, VEGFR-1, VEGFR-2, VEGFR-3, NRP-1 and NRP-2 in the established lymphatic endothelial cells, whereas expression of VEGF-D was not observed (Figure 3). These findings confirm that the endothelial cells that were derived from lymphatic vessels after sentinel lymphadenectomy were completely transferred into the culture system in this study.
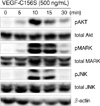
As an initial investigation into the molecular signaling pathways of the isolated lymphatic endothelial cells, it was determined if VEGF-C156S, a mutant form of human vascular endothelial growth factor C that interacts only with the VEGFR-3 receptor in lymphatic vessels (R&D Systems, Minneapolis, USA) induced cell phosphorylation by Akt, MAPK and JNK. Protein phosphorylation was determined by the use of Western blot analysis. The established lymphatic endothelial cells were seeded onto gelatin-coated dishes with EGM-2MV media including 10% FBS. After subconfluent growth, the media was exchanged to EGM-2MV with 3% FBS and cells were cultured at 37℃ overnight. Subsequently VEGF-C156S (500 ng/mL) was added to each dish for 0, 5, 10, 15, and 30 min. Cytosolic protein was isolated from each dish using RIPA buffer with protease and phosphatase inhibitors. The soluble protein was separated on 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and was electrophoretically transferred to nitrocellulose membranes. Following blocking with 5% milk in 0.5% Tween 20 in phosphate-buffer saline, the membrane was probed with primary antibodies (1:2,000 dilution of anti-phosphospecific p44/42 MAPK antibody, anti-phosphospecific Akt antibody, anti-phosphospecific SAPK/JNK antibody; Cell Signaling Technology, Beverly, USA). The membrane was washed and was treated with an anti-rabbit secondary antibody (GE Healthcare, Amersham, UK) at a 1:2,000 dilution. Protein bands were visualized using a commercially available chemiluminescence kit (GE Healthcare, Amersham, UK). The expression of total and phosphorylated Akt, MAPK and JNK protein after treatment of cells with VEGF-C156S for the indicated times is shown in Figure 4. Peak induction of Akt, MAPK and JNK occurred at 10 to 15 min after incubation of isolated lymphatic endothelial cells with VEGF-C156S. Induction returned to the basal level at 30 min.
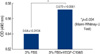
It was examined whether VEGF-C156S induced cell proliferation using an MTS proliferation assay kit (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, USA). A total of 3,000 of lymphatic endothelial cells were seeded in each well of a 96-multiwell plate and were cultured overnight. The cells were treated with or without VEGF-C156S for 48 hr in EBM-2 MV media with 3% FBS. A solution in the MTS assay detection system (20 µL) was added to each well for 3 hr. The absorbance score at a wavelength of 490 nm of each well was measured by the use of a microtiter plate reader.
As shown in Figure 5, effects of VEGF-156S (500 ng/mL) on the proliferation activities were significantly introduced between the 3% FBS condition (MTS score: 0.54±0.0104, n=3) and 3% FBS+VEGF-C156S condition (MTS score: 0.572±0.0061, n=3) as determined by the use of the Mann-Whitney U test (p=0.034).
Since Morton et al.(10) clinically applied an intraoperative identification method of SLN biopsy for malignant melanoma, the importance of characterization of sentinel lymph nodes has been extensively validated for breast cancer and malignant melanoma, mainly in Europe and the United States. In recent years, the treatment method for gastrointestinal cancer has reached a turning point as minimally invasive surgery, including endoscopic surgery, has been on the increase and studies reporting the diagnosis of metastases using an SLN biopsy as an indicator, and the clinical application of SLN biopsies have also appeared.(11-13) The purpose of this study was to develop a novel method for the isolation of anatomically defined lymphatic endothelial cells in order to further investigate the molecular mechanisms of lymphangiogenesis. It has been shown in the present study that human lymphatic endothelial cells can be successfully isolated from sentinel lymphatic channels at the time of the SLN biopsy and it was demonstrated that the cells were definitely derived from lymphatic vessels. A passage culture system was also established. The advantage of this approach is that lymphatic channels are anatomically defined using common surgical techniques including lymphatic mapping and a sentinel node biopsy. The isolation method using collagenase II also enables safe and easy isolation of lymphatic endothelial cells only from the clinical specimen. It was demonstrated that VEGF-C165S induces phospholylated Akt, MAPK and JNK kinase, and the findings suggest that these signaling pathways involving these enzyme may be important in the process of lymphangiogenesis in human lymphatic endothelial cells. Establishment of this procedure will not only help to demonstrate the characteristics of lymphatic endothelial cells in individual cases and the mechanism of lymphatic regeneration in vitro, but establishment of this procedure is also expected to contribute to the exploration of the molecular mechanism of lymphangiogenesis and lymphatic metastases.
Figures and Tables
Figure 1
(A) Image of lymphatic vessel containing blue dye in sentinel lymphadenectomy. (B) A representative micrograph of isolated segment of human afferent lymphatic vessel treated by micro-clips at both ends. (C) A representative micrograph of the afferent lymphatic vessel cannulated centripetally with a sterile silicon tube.

Figure 2
Representative micrographs of immunohistochemical images of VEGFR-3, Prox-1 and Podoplanin in the cultured lymphatic endothelial cells in the collecting lymph vessels (×400).
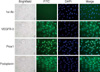
Figure 3
Expression of VEGF families and their receptors by the RT-PCR in our isolated lymphatic endothelial cells.
LECs=lymphatic endothelial cells.

ACKNOWLEDGMENTS
The author thanks all members of Project Research Division, Research Center for Genomic Medicine (Koyama Team) in Saitama Medical University for helpful discussion and advice. This study was supported in part by a Grant-in-Aid for Scientific Research (#16591298, 2003) from the Ministry of Education, Science and Culture of Japan and for Mutual Aid Corporation for Private Schools of Japan (2008). The author partially gave a presentation on this project at the Korean Sentinel Lymph Node Navigation Association (Busan, Korea 2009).
References
1. Ichikura T, Morita D, Uchida T, Okura E, Majima T, Ogawa T, et al. Sentinel node concept in gastric carcinoma. World J Surg. 2002. 26:318–322.

2. Hayashi H, Ochiai T, Mori M, Karube T, Suzuki T, Gunji Y, et al. Sentinel lymph node mapping for gastric cancer using a dual procedure with dye- and gamma probe-guided techniques. J Am Coll Surg. 2003. 196:68–74.

3. Takeda A, Iseki H, Otani Y, Takeuchi H, Ichioka S, Kawai Y, et al. Lymphatic mapping and lymphatic endothelial cell isolation in colorectal cancer patients. Asia Pac J Clin Oncol. 2006. 2:167–174.

4. Detmar M, Hirakawa S. The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med. 2002. 196:713–718.

5. Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001. 20:672–682.

6. Takeuchi H, Takeda A, Iseki H, Shigekawa T, Takahashi N, Andoh T, et al. Isolation and characterization of human lymphatic endothelial cell from primary breast cancer patients. 2007. In : 66th Annual Meeting of the Japanese Cancer Association Program; 144.
7. Mizuno R, Yokoyama Y, Ono N, Ikomi F, Ohhashi T. Establishment of rat lymphatic Endothelial cell line. Microcirculation. 2003. 10:127–131.

8. Garrafa E, Alessandri G, Benetti A, Turetta D, Corradi A, Cantoni AM, et al. Isolation and characterization of lymphatic microvascular endothelial cells from human tonsils. J Cell Physiol. 2006. 207:107–113.

9. Kawai Y, Minami T, Fujimori M, Hosaka K, Mizuno R, Ikomi F, et al. Characterization and microarray analysis of genes in human lymphatic endothelial cells from patients with breast cancer. Lymphat Res Biol. 2007. 5:115–126.

10. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992. 127:392–399.

11. Bilchik AJ, Saha S, Wiese D, Stonecypher JA, Wood TF, Sostrin S, et al. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol. 2001. 19:1128–1136.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download