Abstract
Background
The burden of nontuberculous mycobacterial (NTM) pulmonary disease (PD) is increasing globally. To understand the treatment outcomes and prognosis of NTM-PD, a unified registry is needed. In this project, we aim to construct a multicenter prospective observational cohort with NTM-PD in South Korea (NTM-KOREA).
Methods
The primary objective of this study is to analyze treatment outcomes according to the species. In addition, recurrence rate, adverse events, the impact of each drug on treatment outcomes as well as the impact of characteristics of mycobacteriology will be analyzed. The inclusion criteria for the study are as follows: fulfilling the criteria for NTM-PD having one of the following etiologic organisms: Mycobacterium avium complex, M. abscessus subspecies abscessus, M. abscessus subspecies massiliense, or M. kansasii; receiving the first treatment for NTM-PD after enrollment; age >20 years; and consenting to participate in the study. Seven institutions will participate in patient enrollment and about 500 patients are expected to be enrolled. Participants will be recruited from 1 March 2020 until 19 March 2024 and will be observed through 19 March 2029. During the follow-up period, participants' clinical course will be tracked and their clinical data as well as NTM isolates will be collected.
The burden of nontuberculous mycobacterial (NTM) pulmonary disease (PD) is increasing globally. A population-based study reported the gradual increase of NTM-PD incidence in the United States, from 4.8/100,000 in 2007 to 5.6/100,000 in 20121. In Germany, the prevalence rates of NTM-PD increased steadily from 2.3 to 3.3 cases/100,000 population from 2009 to 2014. These trends have also been observed in South Korea. In that country, the burden of tuberculosis (TB) is intermediate; the recovery rates of NTM according to respiratory specimens and the incidence rates of NTM-PD have been increasing steadily since the early 2000s. Between 2007 and 2016, the incidence of NTM-PD increased from 6.0 cases per 100,000 population to 19.0 cases per 100,000 population2345.
Whereas the epidemiologic significance of NTM-PD has been discussed, its treatment remains complicated. Treatment of NTM-PD requires various combinations of antibiotics for a duration of 18–24 months67. However, standardized regimens for NTM-PD have not yet been fully established67, and the comprising regimens vary, even within the same institution8. Moreover, adherence to the recommended regimens is achieved in only 10% of patients with NTM-PD in actual clinical practice9. The treatment success rates for Mycobacterium avium complex (MAC) and M. abscessus have been reported to be around 60% and 40%, respectively1011.
Under this uncertainty, a unified registry is needed to systematically track patients with NTM-PD. Prospectively merged data might facilitate more consistent evaluation of the epidemiologic features of NTM-PD patients, detailed prescription histories, microbiologic results, and treatment modalities and outcomes. In this project, we aimed to construct a multicenter prospective observational cohort with NTM-PD using an integrated registry system in South Korea.
The objectives of this study are as follows: (1) to develop a multicenter prospective NTM-PD cohort in South Korea, collecting the clinical data of patients with NTM-PD as well as NTM isolates from the time of treatment initiation through the follow-up period; (2) to describe participants' demographic information, comorbidities, initial symptomatic and radiographic features, and microbiologic results at the time of diagnosis; (3) to describe patients' treatment regimens, adverse events, and treatment outcomes; and (4) to analyze the impact of bacteriologic characteristics on prognosis.
Primary objective is analyzing treatment outcomes according to the species and secondary objective are as follows: (1) rates of recurrence (relapse and reinfection, separately); (2) analyzing the impact of the use of each drug on treatment outcomes; (3) analyzing the occurrence of adverse events during treatment; and (4) analyzing the impact of bacteriological factors on prognosis.
The NTM-KOREA is a multicenter, prospective, observational cohort study enrolling patients with NTM-PD in South Korea. After providing their written informed consent, participants will be recruited at seven institutions across South Korea and managed according to the duty physicians' own policy. The participating organizations are Asan Medical Center, Chonnam National University Hospital, Pusan National University Hospital, Pusan National University Yangsan Hospital, Samsung Medical Center, Seoul National University Hospital, and Severance Hospital. The coordinating institution is the International Tuberculosis Research Center. The study will recruit participants from 1 March 2020 until 19 March 2024, and participants will be observed through 19 March 2029.
Participants with all of the following will be included in this project: (1) fulfilling the criteria for NTM-PD, as suggested by the American Thoracic Society/Infectious Diseases Society of America or British Thoracic Society67; (2) having one of the following etiologic organisms: MAC, M. abscessus subspecies abscessus, M. abscessus subspecies massiliense, or M. kansasii; (3) receiving the first treatment for NTM-PD after enrollment (a 4-week interval from treatment initiation to study enrollment is allowed); (4) age >20 years; and (5) consenting to participate in the NTM-KOREA cohort.
Participants will visit their outpatient clinics at about 4- to 12-week intervals. However, the follow-up period could be adjusted according to the duty physicians' clinical judgement. Unplanned visits requested by patients are also allowed. Participants will be observed through the end of the study (19 March 2029).
The below items will be collected.
(1) Demographic information: age, sex, body mass index, smoking status (never/former/current), previous history of TB.
(2) Laboratory findings: white blood cell count (/mm3), hemoglobin (g/dL), platelet count (/mm3), erythrocyte sediment rate (mm/h), total protein (g/dL), albumin (g/dL), total bilirubin (mg/dL), alkaline phosphatase (IU/L), aspartate transferase (IU/L), alanine transferase (IU/L), blood urea nitrogen (mg/dL), creatinine (mg/dL).
(3) Functional capacity and health-related quality of life (HRQL): post-bronchodilator forced expiratory volume at 1 second (FEV1), post-bronchodilator forced vital capacity (FVC), FEV1/FVC, diffusing capacity for carbon monoxide (DLCO), 6-minute walking test, St. George Respiratory Questionnaire and Hospital Anxiety and Depression Scale (HADS).
(4) Comorbidities:
- Respiratory comorbidities: chronic obstructive pulmonary disease, asthma, interstitial lung disease, bronchiectasis, chronic pulmonary aspergillosis, lung cancer, and other respiratory diseases.
- Non-respiratory comorbidities: diabetes mellitus, rheumatologic diseases (including rheumatoid arthritis, systemic sclerosis, and lupus), chronic liver disease, chronic kidney disease, cardiovascular diseases (including myocardial infarction, angina, stroke, transient ischemic attack, pulmonary hypertension and atrial fibrillation), human immunodeficiency virus infection, immunosuppressant use (including transplant recipients) and malignancy (diagnosed within 5 years from the time of enrollment).
(5) Symptoms at the time of treatment initiation: cough (none, present when having upper respiratory infection, frequently coughing or daily coughing), sputum (expectorate everyday, expectorate >4 days per week, expectorate <3 days per week, or rarely), hemoptysis, weight loss (more than 10% of body weight within the past 12 months), and dyspnea (using the Modified Medical Research Council scale).
(6) Radiographic features present on chest computed tomography (CT) scans at the time of treatment initiation (CT scans performed within 3 months before treatment initiation or 1 month after treatment initiation will be reviewed):
- Type: nodular bronchiectatic, fibrocavitary, and unclassified (including consolidation or solitary nodule).
- Presence of cavity: the presence of cavity and the longest diameter on axial view will be collected irrespective of radiographic type. If the number of cavities is two or more, the size will be measured from the largest one.
(7) Bacteriologic results:
- Date of initial diagnosis.
- Source of NTM isolation: sputum/bronchial washing or alveolar lavage/tissue.
- Smear status: negative/trace/1+/2+/3+/4+.
- Etiologic organism: M. avium/M. intracellulare/other MAC/M. abscessus subsp. abscessus/M. abscessus subsp. massiliense/M. kansasii/co-infection.
- Drug susceptibility test: presence of resistance (including inducible resistance) and minimal inhibitory concentration will be provided.
(8) Reasons for treatment initiation:
- Symptomatic aggravation: cough/sputum/hemoptysis/dyspnea/weight loss.
- Radiographic aggravation: bronchiectasis/nodule/consolidation/atelectasis/cavity/ground glass opacity.
(9) Initial treatment regimen:
- Dosing schedule: daily or thrice weekly.
- Initial regimen: clarithromycin/azithromycin/amikacin (intravenous/intramuscularly/inhaled)/rifampin/ethambutol/tigecycline/clofazimine/cefoxitin/imipenem/ciprofloxacin/moxifloxacin/linezolid/other.
- Date of treatment initiation.
- Drugs prescribed at each visit: clarithromycin/azithromycin/amikacin (intravenous/intramuscularly/inhaled)/rifampin/ethambutol/tigecycline/clofazimine/cefoxitin/imipenem/ciprofloxacin/moxifloxacin/linezolid/other.
- Drugs discontinued at each visit.
- Adverse events leading to the discontinuation of drugs: the discontinuation of certain drugs is decided by duty physicians' judgement and will be described on medical records.
- Specific adverse events: nausea/vomiting/diarrhea/azotemia/elevated aspartate aminotransferase or alanine transaminase/hearing impairment/peripheral neuropathy/rash or itching/skin pigmentation/other.
- Mycobacterial culture results.
- Drug susceptibility test results every 6 months if negative culture conversion is not achieved.
- Adoption of adjunctive surgery: wedge resection/segmentectomy/lobectomy (including lobectomy+wedge resection)/lobectomy+segmentectomy/bilobectomy/pneumonectomy/other.
- Recurrence or reinfection, if confirmed.
- Functional capacity and HRQL evaluation (at 6-month intervals): post-bronchodilator FEV1, post-bronchodilator FVC, FEV1/FVC, DLCO, 6-minute walking test, St. George Respiratory Questionnaire, and HADS.
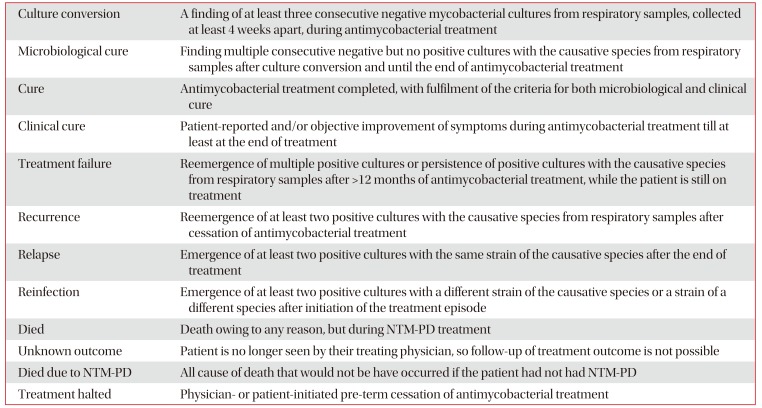
Treatment outcomes will be assessed after completion of the study. The proportion of cured patients (clinical cure and microbiological cure), patients with treatment failure, patients with recurrence, patients with relapse or reinfection, and patients who died will be calculated. The definitions for outcome parameters follow an NTM-NET consensus statement12. The definition for treatment outcomes is in Table 1.
A drug susceptibility test from mycobacterial isolates are generally requested before the initiation of treatment or during the treatment when culture conversion is not achieved or when relapse/reinfection are suspected, according to the current treatment policy. The isolates for which drug susceptibility tests are requested will be stored at the Korea Institute of Tuberculosis (KIT).
The sample size was empirically determined from the experience of our previous cohort study. About 50–70 patients were enrolled in the prospective cohort (NCT01616745) in South Korea each year and half of them received antibiotic treatment. Therefore, at each institute, at least 20 patients could be enrolled in the cohort each year, and the recruiting goal is 100 patients per year across the country.
The ethics approval from each institutional review board will be obtained. This project is conducted in accordance with the principles of Good Clinical Practice.
Data will be collected using a web-based electronic case report form (eCRF) developed by the Medical Research Collaborating Center of Seoul National University Hospital. The eCRFs are collected at the time of enrollment and at follow-up visits at 1-year intervals.
During the study, medical personnel not participating in this investigation will monitor the study. Monitors will visit sites to check all aspects of the study per protocol including adherence to the protocol and Good Clinical Practice, protection of study participants, and data accuracy of the study.
All data are anonymized and secured in the web-based eCRF, operated by the Medical Research Collaborating Center of Seoul National University Hospital. The access to data is restricted to authorized personnel only. Paper files containing participants' data (including personally identifiable information and copies of signed consent forms) will be securely stored in a locked office on-site in locked filing cabinets. Data sharing is possible for stakeholders with the approval of the NTM-KOREA committee.
As the study design is observational and intervention is not allowed, no benefit or harm to participants is expected in this study.
Drop-out from the study is allowed when participants wish to withdraw from the study. These participants will be regarded as “dropped-out”. Patients who die during the study will be counted in the same manner. All data before drop-out will be secured.
The epidemiologic, clinical, radiographic, and microbiological characteristics, as well as treatment outcomes, will be analyzed and compared by subgroup, using the Student t-test, Mann-Whitney test, and Fisher exact test. Multivariable logistic regression will be used to estimate the effect of each drug use and duration on treatment outcomes.
The NTM-KOREA will be the first nationwide observational cohort for NTM-PD in South Korea. In this cohort, seven institutions, distributed across the country, will participate in patient enrollment and about 500 patients with NTM-PD who need treatment are expected to be enrolled. During the follow-up period, up to 10 years, participants' clinical course will be tracked and their clinical data will be collected. Moreover, NTM isolates will be stored for future study.
The relatively low incidence of and lack of an antibiotic pipeline designed for NTM-PD has resulted in NTM-PD being considered an orphan disease13. However, the attention surrounding this neglected disease has increased, and several registries have been launched globally. Registries for NTM-PD have been founded in Italy (ClinicalTrials.gov
NCT02454738 ID: NCT03339063)14 and Singapore (NCT02355015). The noncystic fibrosis bronchiectasis registry, called the Bronchiectasis Research Registry, has recruited participants in the United States and reports that 63% of the population has a history of NTM-PD or NTM infection15. The EMBARC European Bronchiectasis Registry seeks to collect data of NTM-PD as a specific subgroup analysis16. In South Korea, where the incidence of NTM-PD is increasing dramatically2345, this prospective cohort study could provide valuable epidemiological, clinical, and bacteriological data for South Korea.
When participants enroll in the study, their demographic, laboratory, radiographic, and microbiologic information will be collected. Initial treatment regimens will be recorded and changes in microbiological and clinical outcomes will be assessed. Adverse events as well as treatment outcomes are to be collected. In addition, changes in functional capacity and HRQL will be tracked longitudinally. Long-term outcomes after treatment completion will also be analyzed. In particular, differentiation between relapse and reinfection will be made by comparing stored NTM isolates.
The data gathered through NTM-KOREA will provide accurate treatment outcomes, their predictors, and the impact of and adverse events owing to each drug. Follow-up beyond the treatment period will also help in understanding the long-term prognosis of patients with NTM-PD. The information from NTM-KOREA will be the cornerstone of optimizing treatment modalities, duration, and follow-up of patients with NTM-PD in South Korea.
In conclusion, the NTM-KOREA will be launched as the first nationwide prospective observational study of NTM-PD in South Korea. This cohort aims to recruit a total of 500 patients for 5 years, with an additional 5-year observational period. The NTM-KOREA cohort will provide deeper understanding of the treatment outcomes and long-term prognosis in patients with NTM-PD in South Korea.
Acknowledgments
We dedicated this protocol to the late professor Won-Jung Koh, who devoted his entire research career to nontuberculous mycobacterial pulmonary disease.
Notes
Authors' Contributions:
Conceptualization: Kwak N, Choi H, Jeon D, Jhun BW, Jo KW, Kang YA, Kwon YS, Lee M, Mok J, Shim TS, Shin HJ, Whang J, Yim JJ.
Methodology: Kwak N, Yim JJ.
Writing — original draft preparation: Kwak N, Lee M, Yim JJ.
Writing — review and editing: Kwak N, Choi H, Jeon D, Jhun BW, Jo KW, Kang YA, Kwon YS, Lee M, Mok J, Shim TS, Shin HJ, Whang J, Yim JJ.
Approval of final manuscript: all authors.
References
1. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc. 2015; 12:642–647. PMID: 25692495.

2. Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis. 2013; 75:199–204.

3. Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis. 2012; 44:733–738. PMID: 22720876.

4. Lee H, Myung W, Koh WJ, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007-2016. Emerg Infect Dis. 2019; 25:569–572. PMID: 30789139.

5. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 2010; 14:1069–1071. PMID: 20626955.
6. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416. PMID: 17277290.

7. Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017; 72:ii1–ii64.

8. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011; 52:565–571. PMID: 21292659.
9. Adjemian J, Prevots DR, Gallagher J, Heap K, Gupta R, Griffith D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc. 2014; 11:9–16. PMID: 24236749.

10. Kwak N, Park J, Kim E, Lee CH, Han SK, Yim JJ. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis. 2017; 65:1077–1084. PMID: 28582488.
11. Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. Microbiological and clinical outcomes of treating non-Mycobacterium avium complex nontuberculous mycobacterial pulmonary disease: a systematic review and meta-analysis. Chest. 2017; 152:120–142. PMID: 28461147.
12. van Ingen J, Aksamit T, Andrejak C, Bottger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J. 2018; 51:1800170. PMID: 29567726.

13. Cook JL. Nontuberculous mycobacteria: opportunistic environmental pathogens for predisposed hosts. Br Med Bull. 2010; 96:45–59. PMID: 20977990.

14. Aliberti S, Codecasa LR, Gori A, Sotgiu G, Spotti M, Di Biagio A, et al. The Italian registry of pulmonary non-tuberculous mycobacteria - IRENE: the study protocol. Multidiscip Respir Med. 2018; 13(Suppl 1):33. PMID: 30151192.

15. Aksamit TR, O'Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels ML, et al. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest. 2017; 151:982–992. PMID: 27889361.
16. Chalmers JD, Aliberti S, Polverino E, Vendrell M, Crichton M, Loebinger M, et al. The EMBARC European Bronchiectasis Registry: protocol for an international observational study. ERJ Open Res. 2016; 2:00081-2015. PMID: 27730179.

Table 1
List of definitions




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download