Abstract
Background
Methods
Results
Conclusion
Acknowledgments
Notes
Authors' Contributions:
Conceptualization: Butov D, Feshchenko Y, Kuzhko M, Gumenuik M, Butova T.
Methodology: Kikinchuk V, Peshenko A.
Formal analysis: Kikinchuk V, Peshenko A, Butova T, Tkachenko A, Tlustova T.
Investigation: Butov D, Tlustova T.
Writing - original draft preparation: Butov D, Yurko K, Grygorova A, Tkachenko A, Nekrasova N.
Writing - review and editing: Kuzhko M, Gumenuik M, Butova T.
Approval of final manuscript: all authors.
References
Figure 1
Average concentrations of isoniazid in blood serum of patients depending on the time of sample collection. *Differences between independent variables of group 1 and group 2 were considered statistically significant at p<0.05.
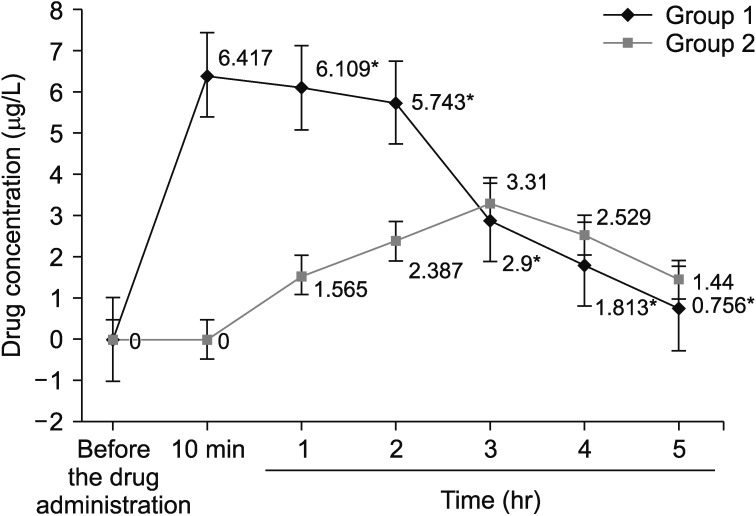
Figure 2
Average concentrations of ethalbutol in blood serum of patients depending on the time of sample collection. *Differences between independent variables of group 1 and group 2 were considered statistically significant at p<0.05.
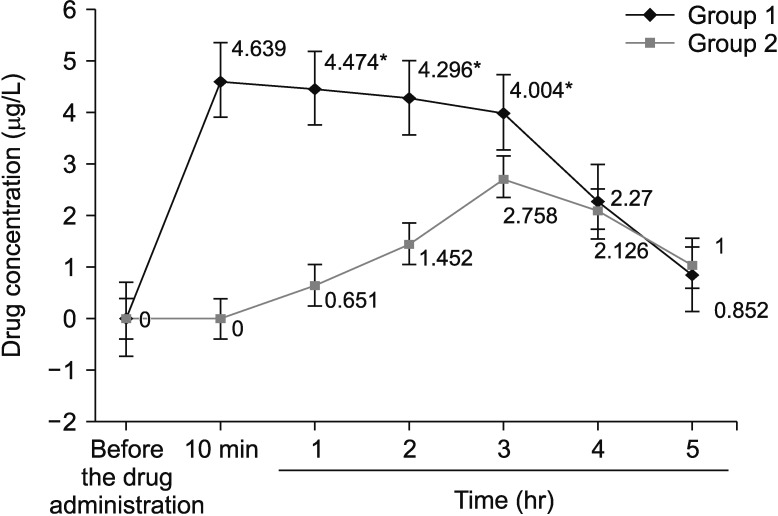
Table 1
Baseline characteristics of patients with TM and HIV infection
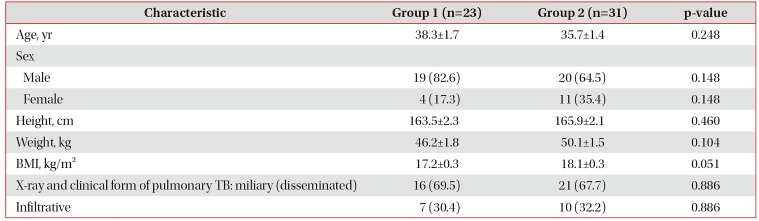
Table 2
Frequency of major clinical symptoms among patients with TM and HIV infection (%)
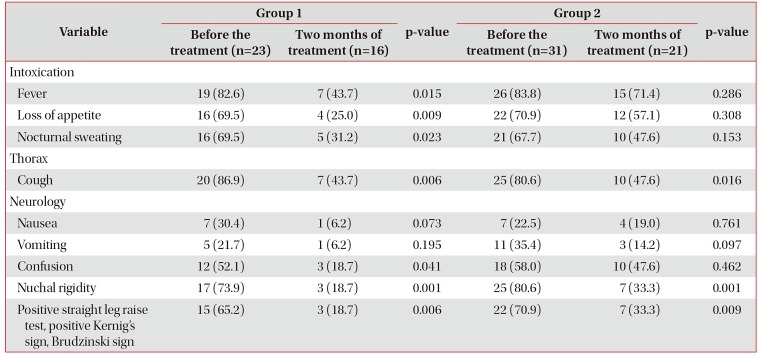
Table 3
Lethal cases in relation to the treatment duration in the examined patients with TM and HIV infection (%)

| Treatment duration | No. (%) | χ2 | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Group 1 (n=23) | Group 2 (n=31) | ||||
| Up to 2 mo | 7 (30.4) | 10 (32.2) | 0.02 | 1.08 (0.34–3.48) | 0.886 |
| Up to 6 mo | 2 (8.7) | 12 (38.7) | 6.194 | 3.80 (1.21–11.90) | 0.016 |
Table 4
Indicators of sputum conversion, evidenced by microscopic and bacteriological sputum examination patients with TM and HIV infection
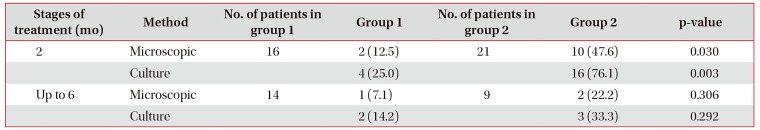
Values are presented as number (%).
Number is the number of sputum MTB-positive patients in each group, excluding the died ones; group 1 included patients with pulmonary TB/TM and HIV infection treated intravenously; group 2 consisted of patients with pulmonary TB/TM and HIV infection treated orally.
TM: tuberculous meningoencephalitis; HIV: human immunodeficiency virus; MTB: mycobacterium tuberculosis; TB: tuberculosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download