Abstract
Background
Efficacy and safety of tiotropium bromide, a muscarinic receptor antagonist, in treatment of asthma have been reported. However, its effect on airway remodeling in chronic asthma of the elderly has not been clearly verified. The objective of this study was to investigate the effect of tiotropium and expression of muscarinic receptors as its related mechanism in an aged mouse model of chronic asthma with airway remodeling.
Methods
BALB/c female mice age 6 weeks, 9 and 15 months were sensitized and challenged with ovalbumin (OVA) for three months. Tiotropium bromide was administered during the challenge period. Airway hyperresponsiveness (AHR) and pulmonary inflammation were measured. Parameters of airway remodeling, and expression levels of M2 and M3 receptors were examined.
Results
Total cell with eosinophils, increased in the OVA groups by age, was decreased significantly after treatment with tiotropium bromide, particularly in the age group of 15 months. AHR and levels of interleukin (IL)-4, IL-5, and IL-13 were decreased, after tiotropium administration. In old aged group of 9- and 15-months-treated groups, hydroxyproline contents and levels of α-smooth muscle actin were attenuated. Tiotropium enhanced the expression of M2 but decreased expression of M3 in all aged groups of OVA.
Conclusion
Tiotropium bromide had anti-inflammatory and anti-remodeling effects in an aged mouse model of chronic asthma. Its effects seemed to be partly mediated by modulating expression M3 and M2 muscarinic receptors. Tiotropium may be a beneficial treatment option for the elderly with airway remodeling of chronic asthma.
The elderly population has increased disproportionately worldwide. It has been predicted that those over 65 years will constitute 20% of the total population in 20301. The proportion of old people who suffer from asthma has increased more than expected previously. In a study of old persons over the age of 60 in Switzerland, the prevalence of physician-reported asthma in old people was found to be around 7%2. In South Korea, a report using national database showed that the prevalence of asthma in the elderly (>65 years old) was 7% in 2013, which was remarkably higher than in other age groups of asthma3. However, elderly asthma is frequently misdiagnosed/underdiagnosed and undertreated due to factors such as frailty, co-morbidities, low perception of their symptoms, poor compliance, and side effects associated with medication and polypharmacy. Older patients diagnosed with asthma exhibits unique features, including severe symptoms, uncontrolled status to standard therapy, and higher mortality4, different from younger asthma patients.
During the aging process, changes in the respiratory tract are largely divided into two categories. The first category is immunosenescence which refers to gradual and various alterations in immune system with age. Its manifestations include imbalances in lymphocyte subset, thymus involution with decreased production of new T cells, defects in apoptosis, mitochondrial function, and malfunction of immune regulatory cells5. These series of age-related changes will increase the susceptibility to infections and develop a status of subclinical, sustained inflammation. The second category is physiologic changes in the lung with aging, including the following: (1) decreased strength of respiratory muscles, (2) decreased lung recoil, and (3) increased stiffness of the chest wall6. Consequently, the elderly takes a breath at higher lung volumes than the young, requiring an additional load on their respiratory muscles. These aforementioned changes associated with aging can affect the development and pathogenesis of asthma in older people.
Acetylcholine is not only a parasympathetic neurotransmitter, but also an autocrine or paracrine hormone in the respiratory tract7. It is also released from non-neuronal cells, including inflammatory, epithelial, and smooth muscle cells. Therefore, in addition to its traditional role in smooth muscle contraction and mucus secretion, it plays proinflammatory, proliferative, and pro-fibrotic roles via interaction with muscarinic receptors. In clinical studies8910, the addition of tiotropium bromide to inhaled corticosteroids (ICS) or ICS plus a long-acting β-agonist (LABA) can also delay initial exacerbation in both in adults and adolescents who have poorly controlled severe asthma. In the 2016 Global Initiatives of Asthma (GINA) report, tiotropium bromide was recommended at steps 4 and 5 as an add-on therapy to the existing treatment for at-risk symptomatic adults with poorly controlled symptoms and forced expiratory volume per 1 second less than 60%11.
In a previous study12, we found a distinct change in the pathogenesis of asthma according to the aging process, including the expression of muscarinic receptors in an acute asthma model. Therefore, the objective of the present study was to investigate the effect of related tiotropium bromide on airway remodeling and the expression of muscarinic receptors in an aged mice model of chronic asthma with airway remodeling.
Three different age groups (6 weeks, 9 and 15 months) of female BALB/c mice (Orient, Seongnam, Korea) were used in this study. To obtain mice with age of 9 and 15 months, six-month-old mice were purchased and raised to the corresponding age in our laboratory. Each age group was divided into three subgroups: control, ovalbumin (OVA), and OVA+tiotropium (5 to 8 mice per group). Based on a previous protocol to make a chronic asthma model13, mice were sensitized by subcutaneous injection with 25 µg of OVA (grade V, Sigma-Aldrich, St. Louis, MO, USA) adsorbed to 1 mg of aluminum hydroxide (Aldrich, Milwaukee, WI, USA) in 200 µL of phosphate-buffered saline (PBS) on days 0, 7, 14, and 21. OVA challenge [20 ng/50 µL in PBS] under isoflurane (Vedco, St. Joseph, MO, USA) anesthesia was done intranasally starting on day 31 twice per week for 9 weeks. Control groups were treated similarly with PBS. Mice were sacrificed at 24 hours after the final OVA challenge. The Animal Subjects Committee of the Catholic University of Korea approved all animal experimental protocols in this study.
Tiotropium bromide was generously donated by Boehringer Ingelheim Co. Ltd. After the sensitization phase, 0.1 mM tiotropium bromide in 50 µL of PBS was administered from day 38 via intranasal nebulization at 30 minutes before the OVA challenge for 5 days a week for a total of 2 months, referring to the previous protocols1314. Control and OVA groups were treated similarly with PBS.
Airway hyper-responsiveness (AHR) was assessed using a flexiVent system (SCIREQ, Montreal, QC, Canada) at 24 hours after the final OVA inhalation based on changes in airway resistance (Rrs; cm H2O/mL/sec) to aerosolized methacholine (Sigma) as described previously15. Under anesthesia, tracheostomized mice were mechanically ventilated at 150 breaths/min and tidal volume of 10 mL/kg with a positive end-expiratory pressure of 3 cm H2O. Mice inhaled saline at baseline and sequentially increasing amounts of methacholine (6.25, 12.5, 25, and 50 mg/mL) via aerosol nebulizer for about 4 minutes at each concentration. Rrs was continuously recorded. Data were shown as peak Rrs value for each methacholine concentration.
To evaluate pulmonary inflammation, total and differential cell counts were determined in bronchoalveolar lavage (BAL) fluid followed by lung histology. Based on our previous protocols1213, after AHR measurement, mice were thoracotomized under anesthesia. BAL fluid was obtained in the right lung through instillation with sterile PBS. After a centrifuge at 353 ×g for 10 minutes at 4℃, supernatants were stored at −70℃. Total and differential cell types such as macrophages, eosinophils, lymphocytes, and neutrophils in the BAL fluid were counted using a hemocytometer. To perform histologic analysis, lungs were processed with 4% paraformaldehyde with overnight incubation and were then cut to sections with thickness of 5 to 6 µm. These sections of the left lung were stained with hematoxylin and eosin (H&E).
Concentrations of type 2 cytokines such as interleukin (IL)-4, IL-5, and IL-13 in supernatants of BAL fluid were measured using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA; BioSource International, Camarillo, CA, USA) according to manufacturers' recommendations.
To evaluate airway remodeling in a chronic asthma model, three crucial indicators (goblet cell hyperplasia, subepithelial collagen deposition, and smooth muscle hypertrophy) were examined. To determine goblet cell hyperplasia, left lung epithelium was stained with periodic acid Schiff (PAS) and quantified with a modified five-point scoring system (grades 0 to 4) of Padrid et al.16. To investigate MUC5AC gene expression involved in mucus hypersecretion in the respiratory tract, total RNA was isolated from lung homogenates with TRIzol (Invitrogen, Grand Island, NY, USA) and reverse-transcribed. Real-time polymerase chain reaction (PCR) was carried out with a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using specific primers and an iQ SYBR gene expression assay (Bio-Rad Laboratories) according to manufacturer's instructions. To evaluate subepithelial fibrosis, total collagen content in the lung was quantified by measuring hydroxyproline content with spectrophotometry. Collagen V was stained immunohistochemically. In collagen V assay, lung samples were incubated with a primary monoclonal antibody against collagen V (Abcam, Cambridge, UK) overnight at 4℃ and then incubated with a biotinylated secondary antibody. The immunoreactivity in the lung tissue was detected using peroxidase reagent and 3-amino-9-ethylcarbazole chromogen. In α-smooth muscle actin (α-SMA) assay, an immunohistology kit (IMMH-2; Sigma-Aldrich) was used to let α-SMA react with a primary antibody. Immunostained area of the lung was quantified using an image analysis system (BX50; Olympus, Tokyo, Japan). It was expressed as per micrometer length of the basement membrane with 150–200-µm-sized internal diameter, referring to our previous protocols1213.
In the lung tissue, protein was quantified using the Bradford assay followed by electrophoresis using 12% sodium dodecyl sulfate-polyacrylamide gels. Following the same method described in the previous studies1213, after protein were transferred to nitrocellulose membrane followed by a blocking process, samples were incubated with the primary antibodies such as polyclonal rabbit anti- mAChR M2 and mAChR M3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then incubated with corresponding secondary anti-rabbit IgG antibodies. Proteins were detected with chemiluminescence system (PRO-MG; DaeSung Company, Korea) and quantified with Image J software (http://imagej.nih.gov/ij)
A comparison between groups was assessed using a t-test or Mann-Whitney U test. Data involving more than two groups were analyzed by one-way ANOVA pairwise and nonparametric Kruskal-Wallis test followed by post-hoc Dunn's multiple comparison test. All values are expressed as mean±standard error of the mean. A p-value less than 0.05 was considered significant. The SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Among OVA groups, the aged mice (9 and 15 months) showed higher Rrs than the younger (6 weeks) during inhalation of methacholine at concentration of 12.5 mg/mL of concentration. In OVA groups at 6 weeks, 9 months, and 15 months, Rrs values were 1.95±0.60, 2.19±0.76, and 2.36±0.64, respectively under 12.5 mg/mL of methacholine, showing no significant differences among groups (p>0.05). Under 25.0 mg/mL of methacholine, these values were 2.30±0.51, 2.82±0.31, and 2.96±1.31, respectively, showing significant differences between 6-week and 15-month groups (p<0.05). Under 50 mg/mL of methacholine, these values were 3.08±0.25, 3.97±1.62, and 3.38±1.77, respectively (p<0.01 between 6-week and 9-month groups). After tiotropium bromide administration, the resistance was decreased significantly in all the three groups irrespective of age (Figure 1).
In the evaluation of cell counts in BAL fluid, numbers of total cells and eosinophils were increased remarkably in OVA groups with increasing age, whereas they showed little differences among control groups. OVA+tiotropium groups showed significant reduction in the total cell number and in eosinophils compared to OVA groups at same age, which was particularly pronounced in aged mice (Figure 2A). H&E staining of lung tissue also showed an increased infiltration of peribronchial inflammatory cells in all OVA groups, which appeared more intense in the aged groups than in the younger group. Tiotropium bromide administration attenuated pulmonary inflammation in all corresponding OVA age groups (Figure 2B). However, few differences were detected in inflammation among control groups at different ages.
With respect to type 2 cytokines, the three OVA age groups showed increased levels of IL-4, IL-5, and IL-13 than their corresponding control groups. In OVA groups, IL-4 and IL-13 levels were the lowest in the 9-month-old group while IL-5 level showed a decreased tendency by increasing age. However, no age-related consecutive pattern of changes was observed (Figure 3). Levels of these cytokines were significantly decreased after administration of tiotropium bromide irrespective of age.
Pivotal structural alterations of airway remodeling in chronic asthma were analyzed in lung tissues, including goblet cell hyperplasia, peribronchial fibrosis, and smooth muscle hypertrophy. Regarding goblet cell hyperplasia, compared with the same-age control groups, all OVA groups showed considerable elevation both in PAS-positive area and in the score regardless of age. However, after treatment with tiotropium bromide, these groups showed significant decline in the score, especially with remarkable changes observed for the 15-month-old group (Figure 4A). The expression of MUC5AC gene in the lung showed a decreased tendency with age in all groups (control, OVA, and OVA+tiotropium groups). Treatment with tiotropium bromide decreased the expression of MUC-5AC in the OVA group at a corresponding age, although the decrease was not statistically significant (Figure 4B). As for peribronchial fibrosis, in the three aged OVA groups, immunohistochemically stained area for collagen V was decreased by tiotropium bromide (Figure 5A). The increase in the hydroxyproline level was higher in older OVA groups than that in the younger OVA group compared to control groups with the same age. Tiotropium bromide decreased the content of hydroxyproline in all OVA groups, especially in the 9-month-old group (Figure 5B). For smooth muscle hyperplasia, immunostained area for peribronchial α-SMA among OVA groups showed a gradual increase with age. Similar age-related patterns of this change were also observed in control groups, although less distinctly. Administration of tiotropium bromide to OVA mice significantly reduced the area of α-SMA compared to the corresponding area in OVA group with the same age. The most significant change was observed in the 15-month-old age group (Figure 6).
In control groups, M3 expression showed an age-related increment. Mice in the 15-month-old group exhibited the highest M3 but the lowest M2 level. However, in OVA groups, no consecutive trend in the expression of M2 or M3 was observed with aging. The 9-month-old OVA group had a unique pattern characterized by the lowest expression of M3 and the highest expression of M2. After treatment with tiotropium bromide, all OVA groups showed an increase in M2 expression and a decrease in M3 expression. A clear suppression of M3 receptor was shown in the 15-month-old-treated group, although the change was not statistically significant (Figure 7).
The current study demonstrated that tiotropium bromide, a muscarinic receptor antagonist, affected airway remodeling as well as airway inflammation and AHR in OVA-induced chronic asthma model not only in 6-week-old mice group, but also in aged, 15-month-old mice group. Furthermore, tiotropium treatment increased the expression of M2 receptor but decreased the expression of M3 receptor in all aged groups, especially with distinct changes of M3 in the oldest OVA group.
The cholinergic system is known to modulate type 2 immune response such as allergic asthma via nicotinic or muscarinic receptors17. In particular, the muscarinic receptors show Th2 polarization by dendritic cells, Th2 differentiation from naive T cells, and activation of mast cells, accentuating allergic response in asthma. Among five muscarinic receptors (M1–M5) in the respiratory tract, M3 subtype is expressed on almost all cell types in the airway. It plays a pivotal role in airway inflammation and remodeling18. Kistemaker et al.19 have reported that only M3 receptor knock-out mice (not M1 or M2 receptors knock-out mice) do not induce any change of airway remodeling, including goblet cell metaplasia, smooth muscle thickening, and collagen deposition after allergen exposure. In this context, tiotropium bromide, a long-acting muscarinic receptor antagonist with a kinetic selectivity for M3 receptors and long half-life of dissociation, is thought to exhibit a strong benefiit in terms of its effect and usage. In a few animal and in vitro experiments1314192021, tiotropium bromide has improved airway inflammation or remodeling in an asthma model. However, all these previous studies were conducted with young aged mice only.
We first investigated how airway remodeling in a chronic asthma model was altered by age and whether tiotropium regulated its pathogenesis in aged mice. Collagen deposition and smooth muscle hypertrophy in airway remodeling of chronic asthma were aggravated more in aged OVA mice (9 and 15 months old) than those in younger OVA mice (6 weeks old). However, differences in these parameters of airway remodeling according to age were less obvious in the control group than those in the OVA group. After treatment with tiotropium bromide, all OVA groups irrespective of age showed an anti-remodeling effect. In particular, smooth muscle hypertrophy was improved siginficantly in 15-month-old OVA mice than that in 6-week-old OVA mice. This result is consistent with a previous study with a Guinea pig model of ongoing asthma by Gosens et al.14. In clinical settings, besides the well-known bronchodilating effect of tiotropium bromide, few clinical studies have addressed its anti-inflammatory or anti-remodeling effects of tiotropium in asthma2223. Hoshino et al.23 have assessed FeNO, an inflammatory marker, and airway wall thickness measured by computed tomography, a remodeling marker, in 53 symptomatic asthmatic patients with ICS/LABA medication after tiotropium treatment for 48 weeks. Although there was no significant difference in the change of FeNO between the placebo and the tiotropium group, they observed that airway thickness was improved remarkably in the tiotropium group, suggesting the anti-remodeling effect of tiotropium in asthma. Further clinical studies are needed to investigate the potential role of tiotropium related with underling mechanisms in asthma.
In pulmonary inflammation, we observed that total cell and eosinophil counts in BAL fluid were increased in a chronic asthma model with advanced age. This finding for OVA groups was in contrast with the results obtained for control groups that showed similar levels irrespective of age. IL-4 and IL-13 showed the highest levels in the oldest aged OVA group in this sutdy. A few previous studies involving acute asthma model122425 have also reported remarkable pulmonary inflammation in older groups than that in younger groups. Franceschi et al.26 have mentioned that inflammaging in the elderly is characterized by age-related chronic, subclinical, and low-grade inflammation27. This proinflammatory state can result in neurodegenerative and cardiovascular diseases that are common in the old population. With increasing age, changes in immune system can result in susceptibility to infection. Such chronic inflammatory condition and vulnerability to infection with age as well as structural alterations in the respiratory tract could contribute to unique features such as severity, frequent exacerbation, and intractability to standard treatments in elderly asthma528.
To verify the underlying mechanism of action by tiotriopium bromide in chronic aged asthma, we investigated expression patterns of M2 and M3 muscarinic receptor subtypes by age and exposure to OVA. Tiotropium administration increased the expression of M2 but decreased the expression of M3, suggesting the pharmacological modulation of those muscarinic receptors, although those changes were not statitstically significant. The inhibitory effect of tiotropium bromide on M3 expression by seemed to be the highest in the OVA group at 15-month-old. Any serial pattern of M2 or M3 expression by age was not observed. On the other hand, our previous study12 has shown that M3 subtype is increased while M2 is decreased in expression with increasing age in the OVA group. A study by Lee et al.29 has shown that expression levels of both M2 and M3 subtypes in smooth muscle cell are higher in young rats than those in old rats. These conflicting results might be due to differences in experimental settings such as the duration of allergen exposure, animals used, and their ages. In the current study, chronic OVA exposure was performed for 3 months to 9 and15 months old mice as well as 6-week-old mice was performed. Further study is needed to determine the expression of muscarinc receptors with age. As for tiotropium bromide, the dose used at 0.1 mM in BALB/c mous was very high, about 50,000 higher than that used in human, despite referring to other studies131430 and overdose could amplify the results. However, different circumstances between animal research and clinical practice including administered methods and self- motivation or not should also be considered. In the present study, intranasal route, not direct inhalation to the lung, and no willingness to have the treatment might remarkably decrease the absorption of tiotropium into the body. Therefore, findings in the present study should be interpretated cautiously and comprehensively.
In conclusion, our study showed that tiotropium bromide, a long-acting muscarinic receptor antagonist, had anti-inflammatory and anti-remodeling effects in a mouse model of chronic, aged asthma. Modulation on expression M2 and M3 muscarinic receptors might be partly involved in the action mechanisms of tiotropium. Our results provide evidence that tiotropium might be effective in the elderly who have chronic asthma with airway remodeling.
Figures and Tables
Figure 1
Effect of tiotropium bromide on airway hyperresponsiveness in a mouse model of chronic asthma with aging: 6-week-old group (A), 9-month-old group (B), and 15-month-old group (C). 6w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide. *p<0.05, **p<0.01, ***p<0.001 compared with the OVA group.

Figure 2
Effects of tiotropium bromide on pulmonary inflammation in a mouse model of chronic asthma with aging. (A) Inflammatory cell counts in bronchoalveolar lavage fluids. (B) Hematoxylin and eosin–stained section of lungs (×200). Values represent mean±standard error of the mean (n=5–8 per group). 6w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide. *p<0.05 and ***p<0.001 compared with the OVA group.
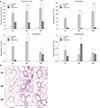
Figure 3
Effects of tiotropium bromide on levels of type 2 cytokines (A, interleukin [IL]-4; B, IL-5; and C, IL-13) in bronchoalveolar lavage fluid in a mouse model of chronic asthma with aging. Values are presented as mean±standard error of the mean (n=5–8 per group). 6w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide. *p<0.05 and ***p<0.001 compared with the OVA group.
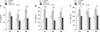
Figure 4
Effect of tiotropium bromide on goblet cell hyperplasia in airway remodeling in a mouse model of chronic asthma with aging. (A) Representative photomicrographs of periodic acid Schiff (PAS)-stained lung sections (×200) and quantification of the PAS-stained area by point scoring as described in Materials and Methods. (B) Expression of mRNA of MUC5AC gene by real-time polymerase chain reaction. 6 w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide. ***p<0.001 compared with the OVA group.
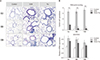
Figure 5
Effect of tiotropium bromide on collagen deposition in airway remodeling in a mouse model of chronic asthma with aging. (A) Immunohistochemical staining for collagen V in lung tissues (×200). (B) Measurement of hydroxyproline content to quantify collagen expression in lung tissues. 6 w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide. **p<0.01 compared with the OVA group.

Figure 6
Effect of tiotropium bromide on smooth muscle hyperplasia in airway remodeling in a mouse model of chronic asthma with aging. (A) Representative photomicrographs of lung sections immunostained α-smooth muscle actin (α-SMA) (×200). (B) Image analysis of areas of immunostaining per micron length of basement membrane in bronchioles. 6 w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide. **p<0.01 and ***p<0.001 compared with the OVA group.

Figure 7
Effect of tiotropium bromide on the expression of M2 and M3 muscarinic receptors in a murine model of chronic asthma with aging. (A) Western blot of M2 and M3 subtypes relative to β-actin in lung tissues. (B) Densitometric measurements for M2 and M3 subtypes. 6w: 6 weeks old; 9M: 9 months old; 15M: 15 months old; OVA: ovalbumin; Tio: tiotropium bromide.

Acknowledgments
This study was supported by a 2014 Grant from The Korean Academy of Tuberculosis and Respiratory Diseases.
We thank Mi-Ran Jo for providing experimental support.
References
1. Cardona V, Guilarte M, Luengo O, Labrador-Horrillo M, Sala-Cunill A, Garriga T. Allergic diseases in the elderly. Clin Transl Allergy. 2011; 1:11.

2. Wuthrich B, Schmid-Grendelmeier P, Schindler C, Imboden M, Bircher A, Zemp E, et al. Prevalence of atopy and respiratory allergic diseases in the elderly SAPALDIA population. Int Arch Allergy Immunol. 2013; 162:143–148.


3. Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean national health database and adult asthma cohort. Allergy Asthma Immunol Res. 2018; 10:387–396.



4. Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, et al. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007; 132:1175–1182.

5. De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis. 2017; 8:162–175.



6. Vignola AM, Scichilone N, Bousquet J, Bonsignore G, Bellia V. Aging and asthma: pathophysiological mechanisms. Allergy. 2003; 58:165–175.


7. Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006; 7:73.



8. Rodrigo GJ, Castro-Rodriguez JA. Tiotropium for the treatment of adolescents with moderate to severe symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2015; 115:211–216.


9. Rodrigo GJ, Castro-Rodriguez JA. What is the role of tiotropium in asthma?: a systematic review with meta-analysis. Chest. 2015; 147:388–396.

10. Cazzola M, Ora J, Rogliani P, Matera MG. Role of muscarinic antagonists in asthma therapy. Expert Rev Respir Med. 2017; 11:239–253.


11. Global Initiative for Asthma. 2016 GINA report, global strategy report for asthma management and prevention. Global Initiative for Asthma;2016.
12. Kang JY, Lee SY, Rhee CK, Kim SJ, Kwon SS, Kim YK. Effect of aging on airway remodeling and muscarinic receptors in a murine acute asthma model. Clin Interv Aging. 2013; 8:1393–1403.

13. Kang JY, Rhee CK, Kim JS, Park CK, Kim SJ, Lee SH, et al. Effect of tiotropium bromide on airway remodeling in a chronic asthma model. Ann Allergy Asthma Immunol. 2012; 109:29–35.


14. Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005; 171:1096–1102.


15. Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, et al. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J Allergy Clin Immunol. 2013; 132:1397–1408.

16. Padrid P, Snook S, Finucane T, Shiue P, Cozzi P, Solway J, et al. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1995; 151:184–193.


17. Bosmans G, Shimizu Bassi G, Florens M, Gonzalez-Dominguez E, Matteoli G, Boeckxstaens GE. Cholinergic modulation of type 2 immune responses. Front Immunol. 2017; 8:1873.



18. Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci. 2015; 36:164–171.


19. Kistemaker LE, Bos ST, Mudde WM, Hylkema MN, Hiemstra PS, Wess J, et al. Muscarinic M(3) receptors contribute to allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2014; 50:690–698.

20. Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007; 30:653–661.


21. Kistemaker LE, Hiemstra PS, Bos IS, Bouwman S, van den, Hylkema MN, et al. Tiotropium attenuates IL-13-induced goblet cell metaplasia of human airway epithelial cells. Thorax. 2015; 70:668–676.


22. Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010; 363:1715–1726.


23. Hoshino M, Ohtawa J, Akitsu K. Effects of the addition of tiotropium on airway dimensions in symptomatic asthma. Allergy Asthma Proc. 2016; 37:147–153.


24. Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007; 37:1392–1403.



25. Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, et al. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann Allergy Asthma Immunol. 2010; 104:236–246.



26. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000; 908:244–254.


27. Ventura MT, Scichilone N, Paganelli R, Minciullo PL, Patella V, Bonini M, et al. Allergic diseases in the elderly: biological characteristics and main immunological and non-immunological mechanisms. Clin Mol Allergy. 2017; 15:2.



28. Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010; 126:690–699.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download