Abstract
Background
Methods
Results
Conclusion
Figures and Tables
Figure 1
Time-dependent phosphorylation of Smad2/3 induced by TGF-β1 5 ng/mL. After stimulation with 5 ng/mL TGF-β1, A549 cells were incubated for the indicated times, and the reactive proteins were electrophoresed on a 10% SDS-PAGE gel. The protein levels of Smad2/3 and p-Smad2/3 were analyzed by western blot. The phosphorylation of Smad2/3 reached a peak 1 hour following TGF-β1 stimulation. TGF-β1: transforming growth factor β1; SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis gel.

Figure 2
Representative results of Smad expression by western blot according to concentration of ATRA (A), 9-cis RA (B), and 13-cis RA (C) stimulation. After stimulation with various concentrations of the three RAs, A549 cells were incubated for 24 hours and then electrophoresed on a 10% SDS-PAGE gel. Expression of Smad2 and Smad3 protein was analyzed by western blot. The optimal concentration of the RAs was 10−6 mol/L, stimulating the maximum expression of Smad2 and Smad3. ATRA: all-trans retinoic acid; RA: retinoic acid; SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis gel.

Figure 3
(A) Representative results of Smad expression by western blot upon pre-stimulation with TGF-β1 followed by administration of each of the three RAs. A549 cells were left untreated (control) or stimulated with one of the following conditions: TGF-β1 alone, each of the three RAs alone, or pre-stimulated with TGF-β1 followed by administration of one of the three retinoic acids. They were incubated for 24 hours and electrophoresed on a 10% SDS-PAGE gel. The levels of Smad2/3 and p-Smad2/3 were analyzed by western blot. TGF-β1 activated Smad2/3 and increased p-Smad2/3. In contrast, RA administration completely inhibited the phosphorylation of Smad2/3. This was similarly observed under conditions of pre-stimulated and simultaneous TGF-β1 administration followed by treatment with the three RAs. (B) Representative results of Smad expression by western blot upon pre-treatment with RA followed by TGF-β1 stimulation. A549 cells were left untreated (control) or stimulated with one of the following conditions: TGF-β1 alone, each of the three RAs alone or pre-treatment with each of the RAs followed administration of TGF-β1. The cells were incubated for 24 hours and electrophoresed on a 10% SDS-PAGE gel. The levels of Smad2/3 and p-Smad2/3 were analyzed by western blot. TGF-β1 activated Smad2/3 and increased p-Smad2/3. RA treatment completely inhibited the phosphorylation of Smad2/3. However, when pre-treated RA was administered, followed by TGF-β1 stimulation, RAs did not suppress the phosphorylation of Smad2/3. TGF-β1: transforming growth factor β1; ATRA: all-trans retinoic acid; RA: retinoic acid; SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis gel.
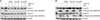
Figure 4
(A–C) Representative western blot results of A549 epithelial cell Smad expression upon pre-treatment with a p38 MAPK inhibitor (B) or a MEK inhibitor (C). A549 cells were pre-treated with a p38 MAPK inhibitor or a MEK inhibitor, and then the cells were stimulated as follows: (1) control, (2) TGF-β1, (3) RA (ATRA), (4) pre-treated with RA (ATRA) prior to TGF-β1 stimulation, or (5) pre-stimulated with TGF-β1 prior to RA (ATRA) treatment. The cells were then incubated for 24 hours and electrophoresed on a 10% SDS-PAGE gel. The levels of Smad2/3 and p-Smad2/3 expression were analyzed by western blot. TGF-β1 did not significantly elevate p-Smad2 expression when epithelial cells were pre-treated with the p38 MAPK inhibitor. When epithelial cells were pre-treated with the MEK 1/2 inhibitor, p-Smad3 expression was not significantly increased by TGF-β1. MAPK: mitogen-activated protein kinase; TGF-β1: transforming growth factor β1; RA: retinoic acid; ATRA: all-trans retinoic acid; SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. *p<0.05, **p<0.01, ***p<0.001.

Figure 5
(A–C) Representative western blot results of CCD-11Lu fibroblast cell Smad expression upon pre-treatment with a p38 MAPK inhibitor (B) or a MEK inhibitor (C). CCD-11Lu cells were pre-treated with a p38 MAPK inhibitor or a MEK inhibitor, and then cells were stimulated as follows: (1) control, (2) TGF-β1, (3) RA (ATRA), (4) pre-treated with RA (ATRA) prior to TGF-β1 stimulation, or (5) pre-stimulated with TGF-β1 prior to RA (ATRA) treatment. CCD-11Lu cells were then incubated for 24 hours and electrophoresed on a 10% SDS-PAGE gel. The levels of Smad2/3 and p-Smad2/3 expression were analyzed by western blot. In fibroblasts pre-treated with the p38 MAPK inhibitor, TGF-β1 did not significantly increase p-Smad2 expression. MAPK: mitogen-activated protein kinase; TGF-β1: transforming growth factor β1; RA: retinoic acid; ATRA: all-trans retinoic acid; SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. **p<0.01, ***p<0.001.

Figure 6
Histological sections of lung fields stained with hematoxylin and eosin (A–F, ×400) and lung injury score (G, H) for the following treatments and at the specified timepoints: control at 1 week (A), bleomycin at 1 week (B), bleomycin followed by ATRA at 1 week (C), control at 3 weeks (D), bleomycin at 3 weeks (E), bleomycin followed by ATRA at 3 weeks (F), lung injury score at 1 week (G), and at 3 weeks (H). Bleomycin-induced lung damage and ATRA attenuates the lung injury. ATRA: all-trans retinoic acid; PBS: phosphate buffered saline; Bleo: bleomycin. *p<0.05, ***p<0.001.
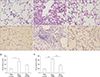
Figure 7
Histological sections of lung fields stained with Masson's trichrome stain and hydroxyproline content in lung tissue. (A) Control at 3 weeks (×400). (B) Bleomycin at 3 weeks (×400). (C) Bleomycin followed by ATRA at 3 weeks (×400). (D) Hydroxyproline content in lung tissue at 3 weeks. Bleomycin-induced lung damage and ATRA attenuates the lung injury. ATRA: all-trans retinoic acid; PBS, phosphate buffered saline; Bleo: bleomycin. **p<0.01, ***p<0.001.

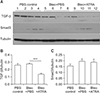
Figure 8
(A–C) Expression of TGF-β and Smad3 in lung lysates upon treatment with bleomycin followed by ATRA, as measured by densitometry at 1 week for TGF-β (B) and Smad3 (C). Male C57BL/6J mice were stimulated as follows: (1) control, (2) bleomycin, or (3) bleomycin followed by ATRA. Bleomycin significantly increased the levels of TGF-β and Smad3, and ATRA significantly decreased the levels of TGF-β and Smad3 at 1 week. TGF-β: transforming growth factor β; ATRA: all-trans retinoic acid; PBS: phosphate buffered saline; Bleo: bleomycin. **p<0.01, ***p<0.001.
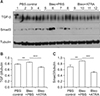
Figure 9
(A–C) Expression of TGF-β and Smad3 in lung lysates upon treatment with bleomycin followed by ATRA, as measured by densitometry at 3 weeks for TGF-β (B) and Smad3 (C). Male C57BL/6J mice were stimulated as follows: (1) control, (2) bleomycin, or (3) bleomycin followed by ATRA. ATRA decreased the levels of TGF-β at 3 weeks but not of Smad3. TGF-β: transforming growth factor β; ATRA: all-trans retinoic acid; PBS: phosphate buffered saline; Bleo: bleomycin. ***p<0.001.
Acknowledgments
Notes
Authors' Contributions
Conceptualization: Lee SH, Park MS.
Methodology: Park MS, Shin JH, Shin MH.
Formal analysis: all authors.
Data curation: Shin JH, Shin MH.
Validation: Lee SH, Park MS, Shin JH, Shin MH.
Investigation: Park MS, Kim YS, Chung KS, Song JH, Kim SY, Kim EY, Jung JY, Kang YA, Chang J.
Writing - original draft preparation: Lee SH, Park MS.
Writing - review and editing: Park MS, Kim YS, Chung KS, Song JH, Kim SY, Kim EY, Jung JY, Kang YA, Chang J.
Approval of final manuscript: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download