This article has been corrected. See "Erratum: Clinical Practice Guideline of Acute Respiratory Distress Syndrome" in Volume 80 on page 95.
Abstract
There is no well-stated practical guideline for mechanically ventilated patients with or without acute respiratory distress syndrome (ARDS). We generate strong (1) and weak (2) grade of recommendations based on high (A), moderate (B) and low (C) grade in the quality of evidence. In patients with ARDS, we recommend low tidal volume ventilation (1A) and prone position if it is not contraindicated (1B) to reduce their mortality. However, we did not support high-frequency oscillatory ventilation (1B) and inhaled nitric oxide (1A) as a standard treatment. We also suggest high positive end-expiratory pressure (2B), extracorporeal membrane oxygenation as a rescue therapy (2C), and neuromuscular blockage for 48 hours after starting mechanical ventilation (2B). The application of recruitment maneuver may reduce mortality (2B), however, the use of systemic steroids cannot reduce mortality (2B). In mechanically ventilated patients, we recommend light sedation (1B) and low tidal volume even without ARDS (1B) and suggest lung protective ventilation strategy during the operation to lower the incidence of lung complications including ARDS (2B). Early tracheostomy in mechanically ventilated patients can be performed only in limited patients (2A). In conclusion, of 12 recommendations, nine were in the management of ARDS, and three for mechanically ventilated patients.
Since the first description of acute respiratory distress syndrome (ARDS) as a series of 12 patients in 1967 by Ashbaugh et al.1, it still remains a major public health problem that incurs high health care costs and causes major mortality in the intensive care unit (ICU) despite improvements in outcomes in the last two decades. ARDS refers to the occurrence of severe hypoxemia that was not corrected by oxygen treatment and is characterized by heterogeneous acute lung inflammation with increased permeability of the alveolar-capillary membrane, resulting in the development of exudate within the alveolar space, damage due to activated neutrophils and cytokines, and abnormalities of surfactant and the coagulation system2. The definition also recently changes as the Berlin criteria3, which was modified to the original American-European Consensus Conference definitions4 and novel clinical trial designs in ARDS may anticipate a new era of successful therapies.
Although over the past decades, there has been a remarkable development in the therapeutic approach and management of critically ill patients with ARDS, the mortality of patients with ARDS is unacceptably high, up to 40%5. In Korea, it has been reported that 79 patients with ARDS were admitted to the ICUs of 28 university hospitals all over the country within 1 month, July 2009, and 45 of those patients died, resulting in a mortality rate of 57%6. Also, until now there is no well-stated clinical practice guideline for intensivists about ARDS, especially focused on the critical care including applying mechanical ventilation until now.
Herein, we report the recommendations and suggestions of how to manage mechanically ventilated patients with or without ARDS.
The board members of the Korean Society of Critical Care Medicine (KSCCM) appointed the editor for the new guidelines addressing ARDS management. The panels of the guideline committee were recruited from the members of the KSCCM and the Korean Academy of Tuberculosis and Lung Diseases (KATRD). The KSCCM and KATRD approved all panelists, and all of them applied voluntarily to the positions. The 16 panelists include intensivists, anesthesiologists, pulmonologists, pediatricians, and methodologists. All of the panelists were required to disclose any conflicts of interest (COI) about the topics. None of the panelists has any COI with the related topic.
The board members of the KSCCM and KATRD agreed with the development of guidelines on ARDS management. During the 2014 KSCCM conference, we surveyed important topics related to ARDS management from the KSCCM members. Initially, 20 topics were collected from the survey. Then, panel members selected 12 topics, with a consensus. All of the panels agreed with the final topics. For each topic, we developed standardized questions using the PICO (population, intervention, comparator, outcome) format.
There was no guideline for ARDS management available during the beginning of the guideline development meeting, and we tried to develop de novo guideline. This guideline is based on Korean AGREE II as an assessment tool, which was published by the Korean Ministry of Health and Welfare and the Korean Academy of Medical Sciences. We ask literature search for a specialist. The National Library of Medicine's medical subject headings (MeSH) keyword nomenclature was used with PICO. We searched the literature using Medline (1948 to July 2014). Searches were limited to literature written in English or the Korean language. We searched all of the possible investigation methods, including retrospective cohort studies and case series. We also searched both original investigations and systemic reviews. We assessed the quality of systemic reviews and original investigations carefully.
The keywords and search formula were based on the PICO elements of the standardized questions and the study design, which is documented in the Korean version (http://www.ksccm.org or http://www.lungkorea.org). Selection of studies was conducted by the specialist of the area. First title screening was completed, then abstract and full-text screening. If a paper was selected for risk of bias assessment, we abstracted the data based on the following characteristics: study design, participants, intervention, control, outcomes, funding, and COI. We assessed the risk of bias using the Cochrane Risk of Bias Tool in randomized trials. We also performed meta-analyses especially in two PICOs, the effect of recruitment maneuvers and neuromuscular blockers. However, the results of the meta-analysis were the same as previously published meta-analyses.
The strength of recommendation as strong or weak was determined based on the value of the study results, wanted vs. unwanted effects, and cost effectiveness910. The strength of recommendation was categorized as strong (grade 1) or weak (grade 2). Each author drafted the recommendations after the entire panels reviewed the evidence and discussed the recommendation. Recommendations were then revised several times during meetings in KSCCM conference rooms, and through email exchanges that included the entire panel.
We used a modified Delphi technique11 to achieve a consensus on each recommendation. This technique aims to minimize any group interaction bias and to maintain anonymity among respondents. The E-mail was exchanged through assistants of KSCCM. Since there was no COI among panelists, all panelists voted on their level of agreement with each recommendation. If a panel disagreed with the draft of a recommendation, the panel suggested a different recommendation. Each panelist provided open-ended feedback on each recommendation with suggested wording edits or general remarks. To achieve a consensus and to be included in the final manuscript, each recommendation had to have an at least 50% agreement (strong or weak) with a response rate of at least 80% of the total panel members. All recommendations achieved consensus during the first round. We repeated a review by all panel members.
External reviewers who were not involved in the development of the guideline had reviewed it before it was published. These reviewers included different academic society members, a methodological expert, and a practicing clinician. The final manuscript was reviewed and approved by the Board of KSCCM and KATRD.
The recommendation and level of evidence of 12 topics were categorized and summarized in Table 1. The details of each topic were as follows.
- We recommend low tidal volume ventilation can be applied to patients with ARDS to reduce mortality (grade 1A).
- The tidal volume should be maintained less than 6 mL/kg of predicted body weight in patients with ARDS.
- The plateau pressure should be maintained less than 30 cmH2O in patients with ARDS.
According to analyses of the causes of death in patients with ARDS, a majority of patients died of multiple organ dysfunction syndromes (MODS) rather than respiratory failure12, and the mortality rate was reported to be significantly higher when another organ failure in addition to respiratory insufficiency, was involved13. ARDS occurs due to various causes, and many kinds of treatments are conducted in individual patients based on the cause. Nevertheless, the fact that MODS is the predominant cause of death has raised the hypothesis that mechanical ventilation, which is essentially and commonly administered to all patients with ARDS, can play a major role in initiating and propagating a systemic inflammatory reaction. This hypothesis is termed ventilator-induced lung injury (VILI), and studies on VILI and its relation to systemic inflammatory response syndrome14 and MODS1516 have been conducted.
The lung of patients with ARDS is characterized by heterogeneous inflammation, with congestion and atelectasis of dependent alveoli and relatively normal alveoli on the opposing side2. In this condition, if mechanical ventilation is applied with 10–12 mL/kg of tidal volume, which is a conventional ventilation strategy, and without positive end-expiratory pressure (PEEP), the physical stretch injury will occur in relatively normal alveoli because of overexpansion. Also, damage occurs from shearing forces, in which the repeated collapse and reopening of the respiration cycle occurs in basal alveoli affected with congestion and atelectasis, and these two injuries are the main mechanisms of VILI17. Barotrauma such as pneumothorax, pneumomediastinum, and subcutaneous emphysema, and volutrauma such as permeability alteration, pulmonary edema, and diffuse alveolar injury, occur via stretch injury. Owing to the shearing force on the site of atelectasis, atelectrauma by repeated alveolar collapse and reopening occurs. In the whole process, biotrauma due to the activation and recruitment of inflammatory cells and mediators occurs. These inflammatory mediators are not limited to the lung, and they enter into the systemic circulation through damaged alveoli-capillary membranes. In infectious lung diseases, bacteria within alveoli can also move into the systemic circulation, causing a systemic inflammation similar to sepsis. Such systemic inflammation causes MODS, along with organ perfusion deterioration due to reduced cardiac output by increased pressure within the thorax induced by mechanical ventilation, leading to the death of patients15. This is the pathological mechanism of VILI and MODS18.
Lung protective ventilation (LPV) strategy refers to a mechanical ventilation strategy to minimize VILI by administering a tidal volume less than the conventional ventilation volume and limiting the plateau pressure to reduce injury to alveoli by increasing end-expiratory lung volume with PEEP19. In a broad sense, prone position ventilation and recruitment maneuvers, which minimize VILI by reducing heterogeneity, and extracorporeal membrane oxygenation (ECMO), which reduces the risk of lung injury from mechanical ventilation and a high concentration of oxygen, can also be included in LPV.
After detailed mechanisms of VILI had been studied and reported, an LPV strategy was conceptualized to prevent VILI, and clinical studies were conducted. In 1998, Amato et al.20 first reported that low tidal volume ventilation had a potential clinical effect on patients with ARDS through a randomized clinical trial. In 53 patients, the conventional ventilation strategy with a tidal volume of 12 mL/kg, low PEEP, and targeting a 35–38 mm Hg partial pressure of carbon dioxide was compared with the protective ventilation strategy with a tidal volume of 6 mL/kg, high PEEP, and permissive hypercapnia. As a result, the protective ventilation group had lower 28-day mortality than the conventional ventilation group (38% vs. 71%), the frequency of barotrauma was low, and weaning rate from mechanical ventilation was higher. However, other clinical studies reported at almost the same time showed that low tidal volume had no clinical effects on patients with ARDS212223, raising controversy over the clinical effect of low tidal volume.
In this circumstance, the ARDS Network study, the most remarkable clinical study related to the clinical effect of low tidal volume ventilation, was reported24. The study was conducted in 10 institutions in the United States for three years on a large scale. A tidal volume of 12 or 6 mL/kg of predicted body weight was applied to 861 patients, and plateau pressures were limited to 50 cmH2O and 30 cmH2O, respectively. The low tidal volume ventilation group showed significantly lower mortality compared to the conventional ventilation group (31% vs. 39.8%, p=0.007). Regarding the indices including days without breathing assistance, ventilator-free days, days without failure of nonpulmonary organs or systems and blood interleukin-6 concentrations, the low tidal volume ventilation group showed significant improvement, providing strong clinical evidence for the effect of low tidal volume ventilation.
According to the Cochrane review on the clinical trials2526, although there was heterogeneity among studies, it was analyzed that 28-day and hospital mortalities were significantly reduced in the low tidal volume ventilation group. Based on the above results of clinical trials and systematic review, the Surviving Sepsis Campaign Guideline which was revised in 2012 recommended low tidal volume ventilation with a high level of evidence27.
Recently, Amato et al.20 reported the result of a multilevel mediation analysis about the nine clinical trials of ARDS. According to the result, driving pressure (ΔP=VT/CRS; VT, tidal volume; CRS, respiratory system compliance) which reflects functional lung capacity is more correlated with the mortality of ARDS patients than tidal volume, plateau pressure, and PEEP. However, this was the statistical analysis of previous studies, and clinical studies should be performed to confirm the clinical effects of ΔP.
- We suggest high PEEP can be applied to patients with ARDS, who have PaO2/FIO2 ≤200 mm Hg to reduce mortality (grade 2B).
- The application of high PEEP does not increase the risk of barotrauma.
- If high PEEP is applied, PaO2/FIO2 at day 1 and three can be improved compared to the application of low PEEP group.
PEEP is an easily applicable intervention and an essential component of the care of critically ill patients who require ventilator support. The end-expiratory pressure is elevated above atmospheric pressure to prevent atelectasis and correct the hypoxemia caused by alveolar hypoventilation. Mechanisms that PEEP improves through gas exchange and pulmonary function are increased functional residual capacity, alveolar recruitment, redistribution of extravascular lung water and improved ventilation-perfusion matching28. Also, it may prevent repetitive alveolar collapse. High PEEP has been defined differently in each study. In ALVEOLI study29, it was based on PEEP table, and in EXPRESS study30, PEEP was raised as plateau pressure reached 28–30 cmH2O. It can be expected that it may reduce VILI with the above physiological effect. However, side effects include an increase in physiologic dead space, decreased cardiac output, and increased risk of barotrauma313233.
A randomized clinical trial that applied high PEEP was conducted with 53 patients with early ARDS20. In the group that was exposed to high PEEP and low tidal volume, mortality on day 28 (38%) was significantly lower than the conventional ventilation group (71%), but there was no significant difference in survival to hospital discharge. In this study, mortality of conventional ventilation group was too high, and the effect of high PEEP was observed on day 3. In a second randomized study, ICU mortality, hospital mortality, and ventilator-free days at day 28 all favored the high PEEP group34.
According to the Cochrane's review, which meta-analyzed seven randomized control trials20293034353637 conducted from 1998 to 2009 to compare the effects of the applications of low PEEP and high PEEP, there was heterogeneity that five2930353637 out of seven included trials had the application of the same tidal volume in both high and low PEEP groups, whereas two studies2034 had different tidal volumes for the two groups. Therefore, as a result of analyzing 2,565 patients with ARDS, who were administered the same tidal volume with different PEEP, high PEEP did not contribute to the reduction of mortality in the hospital (relative risk [RR], 0.90; 95% confidence interval [CI], 0.81–1.01). In the group with PaO2/FIO2 ≤200 mm Hg, high PEEP decreased mortality within the ICU (RR, 0.67; 95% CI, 0.48–0.95). The comparison between the two groups on barotrauma did not show a significant difference (RR, 0.97; 95% CI, 0.66–1.42). However, PaO2/FIO2 at days 1 and 3 was improved in the high PEEP application group. In a follow-up study38 of the two large-scale studies3036 in 2014, the group in which PaO2/FIO2 increased by more than 25 mm Hg within 2 hours by applying PEEP showing decreased mortality (31% vs. 51%; odds ratio [OR], 0.8; 95% CI, 0.72–0.89). Also, the reduced mortality was observed in patients with increased PaO2/FIO2 regardless of PEEP among ARDS patients with PaO2/FIO2 ≤150 mm Hg.
If PEEP recruits collapsing alveoli, atelectrauma of alveoli can be reduced39 whereas alveolar injury may be increased by raising the intensity delivered to alveoli if they are not recruited40. It is expected that further clinical trials will apply different PEEP to patients with enough alveoli to be recruited. Based on the above data, high PEEP can be applied to ARDS patients with PaO2/FIO2 ≤200 mm Hg.
- We recommend prone position can be applied to patients with moderate or above ARDS to reduce mortality if it is not contraindicated (grade 1B).
- The prone position should be applied when there is no improvement of oxygenation at an early stage of mechanical ventilation.
- Prone position is recommended at least for 10 hours.
- Lung protective strategy should also be applied during prone positioning.
Based on the theory that the use of the prone position would reduce lung injury caused by lung stress and strain exerted on the lungs during artificial ventilation in ARDS414243 since its first attempt by Bryan in 197444, studies have been performed to prove its utility by a number of researchers. In a post hoc analysis of Gattinoni et al. in 200145, among PaO2/FiO2 <88 mm Hg, Simplified Acute Physiology Score II (SAPS II) ≥49 and tidal volume >12 mL/kg of predicted body weight groups, the group that used the prone position had a lower 10-day mortality45, and other meta-analyses reported that the patients using the prone position in the case of PaO2/FiO2 <100 mm Hg had lower mortality464748, but there were a lot of controversies over the insistence of the use of the prone position can decrease mortality in ARDS. However, a large-scale randomized study has recently been conducted by Guerin et al.49, reporting that in the PaO2/FiO2 <150 mm Hg group at FiO2 >0.6 and PEEP 5 cmH2O, the group that conducted the prone position for at least 16 hours per day within 36 hours had a statistically significant decreased 28-day mortality rate (16.0% prone group vs. 32.8% supine group: hazard ratio [HR], 0.39; 95% CI, 0.25–0.63; p<0.001) and 90-day mortality (23.6% prone group vs. 41.0% supine group: HR, 0.44; 95% CI, 0.29–0.67; p<0.001) compared to the group with the supine position, despite the mechanical ventilation for 12 or 24 hours49. In the meta-analysis conducted after the publication of the large-scale study by Guerin et al.49, the use of the prone position was found to decrease mortality of patients with moderate ARDS505152. However, patient's severity, low tidal ventilation, time of prone position use, and the degree of PEEP were different in each study, showing significant heterogeneity415052. When using a low tidal volume ventilation concomitantly, the mortality was decreased with a statistical significance (RR, 0.66; 95% CI, 0.5–0.85; p=0.02)4953545556, but the group without low tidal volume ventilation did not show decreased mortality (RR, 1.00; 95% CI, 0.88–1.13; p=0.949)56. The time of using the prone position showed differences concerning mortality. When the prone position was conducted for over 10 hours, a clearly decreased mortality was found (OR, 0.62; 95% CI, 0.48–0.79; p<0.001), and the randomized study by Taccone et al.53 and Mancebo et al.54, as well as meta-study by Hu et al.51 and Beitler et al.56, reported that the decrease in mortality was evident when the prone position was conducted for 12 hours or more. In the large-scale randomized study conducted by Guerin et al.49, the prone position was performed for more than 19 hours4951535455. However, when the prone position period was less than 12 hours, there was no decrease in mortality (OR, 1.04; 95% CI, 0.80–1.36; p=0.757). No reduction in mortality was shown when analyzing patients with acute lung injury or mild ARDS (OR, 1.02; 95% CI, 0.76–1.36; p=0.920)50. Although there were not many studies on the degree of PEEP, the meta-analysis published by Hu et al.51 reported that the group maintaining high PEEP ≥10 cmH2O–13 cmH2O showed a lower 60-day mortality (RR, 0.82; 95% CI, 0.68–0.99; p=0.04) and 90-day mortality (RR, 0.57; 95% CI, 0.43–0.75; p<0.0001), compared to the group maintaining PEEP <10 cmH2O when conducting prone position.
In randomized clinical trials and meta-analyses, the use of the prone position was reported to improve hypoxemia. Compared to the group using the supine position, the prone position group showed an increase of PaO2/FiO2 by 25%–36% for the first 3 days444552, which contributed to the improvement of perfusion imbalance by reducing the collapse of the dependent portion of the lung, as well as edema415758.
In three randomized clinical trials, there was a study on mechanical ventilation period535559, showing no difference in the mechanical ventilation period between the prone position and supine position patient groups. In two randomized clinical trials, the result of ICU length of stay showed no difference between the prone position and supine position patient groups5355.
In the study of Guerin et al.49, patients were ventilated while in the prone position for more than 19 hours. A meta-study by Lee et al.50 reported that the decrease in mortality in the prone position for more than 10 hours was statistically significant, and randomized study of Taccone et al.53 and Mancebo et al.54, and meta-study of Hu et al.51 and Beitler et al.56 showed that the decrease in mortality was clear when using the prone position for more than 12 hours4951535455. However, if the duration of the prone position is less than 10 hours, there was no decrease in mortality (OR, 1.04; 95% CI, 0.80–1.36; p=0.757)50. Studies495053545556 showed different indications regarding the discontinuation time when conducted in the prone position. In Guerin et al.49, the prone position was discontinued when (1) there was improvement of hypoxemia (PaO2/FIO2 ≥150 mm Hg with PEEP ≤10 cmH2O and FIO2 ≤0.6), (2) when PaO2/FiO2 deteriorated by 20% or more compared to the supine position, and (3) when complications occurred due to the prone position. It is thought that further studies on daily use time and total treatment period for the prone position will be needed.
The complications related to prone position include extubation, endotracheal tube obstruction, selective main bronchus intubation, pneumothorax, cardiac arrest, arrhythmia, loss of venous or arterial access, increased pressure ulcers, pneumonia related to mechanical ventilation, and increased use of sedatives415456. Rarely, there was no statistical difference of optic nerve injury, retinal scarring, cardiac arrest, loss of arterial access, and pneumonia related to mechanical ventilation between prone and supine position patient groups50. There are contraindications for the use of prone position4149; (1) patients with intracranial pressure >30 mm Hg or cerebral perfusion pressure <60 mm Hg, (2) patients with massive hemoptysis, (3) patients who have received tracheal surgery or sternotomy within 15 days, (4) patients with head injuries within 15 days, (5) patients who had deep vein thrombosis within 15 days, (6) patients who have received an inserted cardiac pacemaker within 15 days, (7) patients with spine, femur, or pelvis fracture, (8) patients with mean arterial pressure ≥65 mm Hg, (9) pregnant women, (10) patients with thoracic duct in precordial region, and (11) patients with abdominal open wound. In the randomized study conducted by Guerin et al.49, patients with ECMO were subjects to be excluded, but in recent studies, the use of prone position after performing EMCO without complications was reported. Especially, regarding the loss of ECMO was difficult in patients who received venous ECMO, some cases reported a successful loss of ECMO using the prone position60616263.
- We suggest ECMO as a rescue therapy in patients with ARDS without improvement of hypoxia by LPV strategy (grade 2C).
Since the first application of ECMO in patients with severe hypoxia and respiratory failure in 197264, two randomized clinical trials6566 have been performed, both group of ECMO and control which showed only a high level of mortality and failed to show any difference in mortality. However, the results of a number of prospective observational studies published after the development of ECMO at the end of the 90s6768 showed the survival rate of ARDS applied by ECMO continued to increase, and in patients with H1N1 influenza-ARDS receiving ECMO, particularly, a high survival rate was found (RR, 0.45; 95% CI, 0.26–0.79; p=0.008)67. On the other hand, in the third randomized clinical trial conducted in 2009, ECMO showed a statistically significant difference in death or disability at 6 months in patients with adult influenza-ARDS patients with severe respiratory failure (RR, 0.69; 95% CI, 0.05–0.97; p=0.03), but did not show a significant decrease in mortality regarding the mortality itself at 6 month or before discharge (RR, 0.73; 95% CI, 0.52–1.03; p=0.07), along with many limitations in methodology of study6970. In addition, a recently conducted meta-analysis mentioned that the effect of the application of ECMO in patients with severe ARDS cannot be concluded in the current situation because of the effect on the improvement of mortality was not statistically significant (OR, 0.71; 95% CI, 0.34–1.4; p=0.358)71. Therefore, the application of ECMO in patients with ARDS must be limitedly or selectively performed, especially for severe ARDS patients, after considering financial and ethical issues thoroughly until the ongoing large-scale randomized study result19 comes out. Regarding this, a cohort analysis68 in 2013 reported that prognosis would be bad if age, lactate level, and plateau pressure before the application of ECMO were high. A domestic retrospective observational study72 has shown that survival rate tended to be high if relatively early ECMO is conducted in young patients.
In patients with ARDS, among mechanical ventilation applied by low tidal volume and enough PEEP, ECMO may be tried as a salvage therapy if low tidal volume ventilation cannot be maintained because of persistent hypoxemia or intolerable hypercapnia73. However, the cause of ARDS should be reversible, or lung transplantation should be possible, and especially the period for the application of mechanical ventilation should be at least within seven days before considering ECMO for ARDS. Also, patients must not be in irreversible multi-organ failure or end stage cancers at the time of receiving ECMO. Patients should also not be in the condition of irreversible central nervous disorders. Particularly, there may be limits of use in patients with current acute bleeding or a high bleeding tendency because the use of a lot of anticoagulants will be needed during ECMO70. Regarding appropriate patient selection, the application of Respiratory ECMO Survival Prediction Score (RESP-score) which is derived from the recent the Extracorporeal Life Support Organization (ELSO) data analysis may be considered, but it is not possible to be considered as an absolute indication74. Urgent studies should be conducted to evaluate the possibilities of domestic application of such prognosis precursors.
The complications related to ECMO can be largely divided into the complications related to patients and related to the device. According to the data published by ELSO70, complications related to patients were reported to be complications related to infection identified for culture (18%), followed by bleeding in the catheterization site (15%), and bleeding in the operation site (14%). Regarding the complications related to the device, the blood coagulation in oxygenation device was reported to be most common with 20%. To prevent such complications, multi-disciplinary team related to ECMO should be made to conduct continuous education and simulation programs. It is also important to monitor patients, the device, and the whole team constantly. As the long-term prognosis of patients who receive ECMO is not known yet, further studies about this are also needed.
Currently, the technology of ECMO is developing very quickly, and the new membrane oxygenator and catheter, which are not available in Korea, are being developed. Also, the need for experienced centers in which ECMO can be conducted skillfully is constantly rising. Although it is true that the application of ECMO is explosively increased through the experience in ARDS accompanied to H1N1 influenza, there are still some problems, including selecting adequately applicable patient groups, optimal catheter composition, and method, appropriate mechanical ventilation and cost-effectiveness during ECMO. Therefore, until results of some well-planned randomized studies come out, the application of ECMO in patients with ARDS may be conducted relatively early only if there is no improvement after applying known LPV strategies such as PEEP, prone position, and alveolar recruitment maneuver.
- We suggest recruitment maneuver can be applied to patients with ARDS to reduce mortality (grade 2B).
- Recruitment maneuver has an effect on improving hypoxia, without increasing the risk of barotrauma.
Recruit maneuver (RM) can prevent repeated opening and closing by opening the collapsed alveoli. Also, if the collapsed alveoli are opened by RM, the lung volume with overall ventilation will be increased to distribute the same amount of tidal volume to more alveoli, resulting in the reduction of alveolar overdistention in patients with ARDS as well. There are some methods of RM, but the one that uses airway pressure is most commonly used. Usually, RM is conducted so that pressure is applied maintain airway pressure at 35–45 cmH2O for 30–40 seconds. In the past, the method to reach the target pressure by exerting a large volume once in the middle of respiration was used75. Recently, the method where the target pressure is obtained by gradually increasing PEEP and decreasing pressure support is also used76.
The existing RM studies showed a significant improvement of hypoxia after RM7778. According to a recent meta-analysis79 and the results of our analysis on RM studies, a statistically significant decrease in mortality was observed in RM group compared to the control group. However, because each study is different with the risk of bias, and there is a possibility that other ventilator interventions conducted along with RM affected the outcome, it must be careful to interpret the result. There was no significant difference of barotrauma between RM and control groups777879.
Low blood pressure often occurs during RM, but most cases are recovered after RM. It is thought that it is because the lung is distended to reduce preload and increase afterload of the right ventricle in RM. A temporary hypoxia may occur during RM because alveoli that are already opened are overexpanded by high airway pressure to press adjacent blood vessels, reducing the perfusion of alveoli with good ventilation. However, most instances of hypoxia will be improved when the airway pressure is reduced after RM. Furthermore, if collapsed alveoli are opened because of successful RM, overall hypoxia will be improved, and hypoxic vasoconstriction will also be reduced to decrease the afterload of the right ventricle. Due to high airway pressure in RM, barotrauma is concerned. Fortunately, according to existing prospective studies, the occurrence of pneumothorax is reported to be very small7778.
The groups expected to have good RM will now be discussed. Patients with ARDS in the early "exudative" phase are more likely to react with RM than the ones in the late "fibrotic" phase80. Extrapulmonary ARDS is more likely to react with RM than pulmonary ARDS8182. The case in which ARDS is diffused is more likely to succeed in recruitment than the locally diffused case, and the effect of RM drops when baseline PEEP before RM is high82. Severe ARDS rather than moderate ARDS is more likely to be recruited.
- The use of systemic steroids cannot reduce mortality in patients with ARDS (grade 2B).
- In the case of a low dose of systemic steroid is used in the early stage, it may improve hypoxemia and reduce the period of mechanical ventilation, the length of ICU stay, and mortality.
Because systemic steroids have strong anti-inflammatory and anti-fibrotic effects in patients with ARDS, it has been considered an effective treatment. Based on this idea838485, seven randomized control trials have been conducted from 1985 to 200786878889909192. However, these trials reported different results regarding the effect of the use of steroids for the reduction of mortality in patients with ARDS. In 1998, Meduri et al.89 reported that mortality was decreased as a result of administering methylprednisolone 2 mg/kg in patients with unresolved ARDS within seven days after artificial intubation for 32 days (12.5% for corticosteroids vs. 62.5% for placebo, p=0.04). Based on this, ARDS Network conducted the Late Steroid Rescue Study (LaSRS)90. In this study, mortality was compared after administering methylprednisolone 2 mg/kg in patients with ARDS, which passed 7 days or more after artificial intubation for 25 days. Here, the 60-day mortality (29.2% for corticosteroids vs. 28.6% for placebo, p=1.0) and 180-day mortality (31.5% for corticosteroids vs. 31.9% for placebo, p=1.0) could not be reduced, but the 60-day mortality of patients whose artificial intubation passed 14 days was reported to be increased (35% for corticosteroids vs. 8% for placebo, p=0.02). A meta-analysis of randomized and cohort studies also showed conflict results93949596979899. There were studies that supported that steroids did not affect the improvement of mortality9495 whereas other studies suggested that the use of low dose of steroids (≤methylprednisolone 2 mg/kg) within 14 days after the occurrence of ARDS might show improvement on short-term mortality9699. However, because the studies included in the analysis contain cases with different administration doses of steroids, the severity of disease, the cause of lung injury, starting a period of steroid administration, and application method of the mechanical ventilation, which make it difficult to conclude the effect of the administration of steroids.
In the LaSRS, it was reported that the use of steroids (methylprednisolone 2 mg/kg) in patients with ARDS within seven days or more of the disease period would improve hypoxemia90. Further, Meduri et al.89 (methylprednisolone 2 mg/kg) in 1998 reported that the use of steroids improved hypoxemia in patients with ARDS (PaO2/FiO2 of 262 for corticosteroids vs. 148 for placebo; p<0.001), and Confalonieri et al.100 reported that PaO2/FiO2 showed statistically significant improvement in the group that was administered steroids (hydrocortisone 200 mg) (p=0.0007). In the same study, the use of steroids in patients with ARDS with 7 days or more of a disease period improved respiratory elastance and blood pressure and reduced the days of application of a respirator for the first 28 days, shock continuation days, and ICU hospitalization period90. In Meduri et al.91 and Annane et al.92, there were reports that it reduced the days of application of mechanical ventilator909192 and ICU hospitalization period91.
Weigelt et al.86, who used a high dose of steroids (methylprednisolone 30 mg/kg every 6 hours for 48 hours)86 reported that infection rate increased in the group using steroids, and a systemic review of Lamontagne et al.97 also reported that the risk of infection would increase when using a high dose of steroids (RR, 1.77; 95% CI, 1.23–2.54). However, Annane et al.92 (hydrocortisone 50 mg every 6 hours) or Meduri et al.91 (methylprednisolone, 2 mg/kg), and the LaSRS reported that the use of low-dose steroid therapy would not increase infection rate899192. However, because the definitions of the secondary infection related to steroids were different each other, screenings were not properly conducted, and the number of patients was small, it is still controversial whether the secondary infection rate will be increased if steroids are used in patients with ARDS.
When steroids are used in patients with ARDS, steroid-related neuromyopathy makes the body unable to move. When a neuromuscular blocking agent is used, the action of a glucocorticoid will be reinforced. If sepsis is involved, it was considered to occur as it promoted degradation of myoprotein. In the LaSRS, neuromyopathy occurred in nine patients, and all of them occurred in the group using steroids (p=0.001). However, other studies reported that the use of steroids did not increase myopathy with statistical significance (Meduri et al.91: 6.4% for corticosteroids vs. 3.6% for placebo, p=1.0; Confalonieri et al.100: 0% for corticosteroids vs. 13% for placebo, p=0.001)91100. Since there is a controversy over steroid-related myopathy because of insufficient studies on it, further studies will be needed. Complications including gastrointestinal tract bleeding, hyperglycemia, other major organ failures (heart, kidney, and liver), arrhythmia, pneumothorax, and psychiatric disorders were reported, but their incidence did not show a statistically significant increase in patients with the steroid administration group909192100. Besides, there was a report that patients in the steroid administration group had a higher frequency of using the respirator again after extubating endotracheal tube90.
- We suggest using neuromuscular blockade for 48 hours after starting mechanical ventilation in patients with ARDS (grade 2B).
- The use of neuromuscular blockage in patients with ARDS has an effect on improvement of hypoxemia for first 48 hours.
- The use of neuromuscular blockage can reduce barotrauma such as pneumothorax in patients with ARDS.
Neuromuscular blockage can maintain transpulmonary pressure appropriately and reduce lung injury related to mechanical ventilation by reducing asynchrony of ventilator and patients' respiration in ARDS101. Including prospective studies related to neuromuscular blockage102103 and a retrospective study related to acute respiratory failure104, meta-analyses showed the effect of neuromuscular blockage on mortality reduction with great effect (OR, 0.78; 95% CI, 0.39–0.90). Additionally, an observational study showed that the early use of neuromuscular blockage had an effect on reducing mortality in severe sepsis caused by the respiratory system104. However, to confirm the daily use of neuromuscular blockage in ARDS, more studies including larger scaled clinical trials are still required105.
Neuromuscular blockage can reduce elastance of the thorax to relieve ventilation/perfusion imbalance in ARDS. In a retrospective study, the use of neuromuscular blockage for 48 hours had an effect on improving hypoxemia up to day 5101. In a meta-analysis, however, the effect that hypoxemia was significantly improved at 48 hours after beginning the treatment with neuromuscular blockage when compared to the control group106. Although the mechanism improving hypoxemia is not clear, it is thought that synchrony between patient respiration and mechanical ventilation will be improved to change lung compliance and gas exchange for the better. Also, the mechanism is thought to reduce barotrauma and atelectasis in expiration by controlling inhaled air volume and pressure in lung inflammatory reaction, consequently resulting in the reduction of lung and systemic inflammations107. Although it can be expected that the duration of mechanical ventilation will be increased because of weakened neural muscles due to neuromuscular blockage, the meta-analysis showed that it was not different from the control group. However, successful ventilator-free days were longer in the group using neuromuscular blockage by comparing the risk of death and period of mechanical ventilation106.
Although neuromuscular blockage is known to be related to weakness generated in ICU, the weakness in patients with ARDS who used it for 48 hours did not increase significantly when compared to the control group in the meta-analysis106. The method to measure weakening of neural muscles after using neuromuscular blockage is possible to decrease sensitivity and specificity of diagnosis based on quadriplegia that may be clinically measured101102. However, a recent study evaluated the weakening of muscles based on Medical Research Council scores108, and if the weakening of muscles is not discovered is considered, it can be thought that the use of neuromuscular blockage in ARDS for less than 48 hours did not significantly increase weakening of neural muscles. This is different from the long-term muscle weakening that occurred after using neuromuscular relaxant in patients with asthma and sepsis in the past109110.
When the neuromuscular blockage is used in patients with ARDS for 48 hours, barotrauma such as pneumothorax was significantly reduced compared to control group. In three retrospective studies, comparative risk (RR, 0.43; 95% CI, 0.20–0.90; p=0.02; I2=0), indicated a lesser degree of barotrauma when compared to the control group106.
If a patient has a renal or liver function disorder as well as cardiovascular problems, the careful selection of neuromuscular blockage will be needed. Patients with normal renal and liver functions prefer pancuronium, and the ones with renal or liver function disorders prefer atracurium or cisatracurium. In cases of the cardiovascular problem, vecuronium is known to be hemodynamically stable with the least amount of side effects111112.
- The use of high-frequency oscillatory ventilation (HFOV) should not be recommended as a standard treatment method in adult patients with ARDS (grade 1B).
- HFOV does not improve survival in patients with ARDS.
- HFOV may cause side effects such as barotrauma or low blood pressure.
As a result of meta-analysis, the application of HFOV in patients with ARDS had no difference, compared with conventional mechanical ventilation, in the 30-day or overall hospital mortality61113114. Rather, based on the result of studies that were terminated because of significantly increased the mortality of patients with ARDS (RR, 1.33; 95% CI, 1.09–1.62; p<0.01), the regular application of HFOV in patients with ARDS is not recommended115116. When applying a LPV strategy, particularly, it seems there is no additional benefit of HFOV. In a meta-analysis114 and randomized control trials115116117118, there was no difference in the duration of mechanical ventilation between HFOV and conventional mechanical ventilation. Because HFOV maintains very small tidal volume and high mean airway pressure, the improvement of oxygenation can be expected. In a meta-analysis excluding children113, the improvement effect of oxygenation (PaO2/FIO2) by HFOV continued from the first 24 hours to three days, but it was not identified in the case of including children61. Therefore, it is hard to conclude that HFOV has an effect on the improvement of oxygenation. Also, even with the improvement of oxygenation, the causes of lung injury or ARDS will not be improved, and most patients with ARDS died of multiple organ failure119. Therefore, temporary improvement of oxygenation seems difficult to be connected to the improvement of survival rate120.
In a meta-analysis, the application of HFOV tended to increase hemodynamic instability such as barotrauma and low blood pressure, but it was not statistically significant. Such side effects showed that higher mean airway pressure in patients who receive HFOV up to 3 days113, which resulted in decreased venous return by intrathoracic pressure and increased right ventricular afterload121122.
- The use of inhaled nitric oxide (iNO) should not be recommended as a standard treatment method in adult and child patients with ARDS (grade 1A).
- The use of iNO in patients with ARDS may increase the risk of renal injury in adults.
As a result of meta-analyses, the use of iNO in patients with ARDS showed no significant reduction in mortality when compared to the control group, regardless of the severity of hypoxemia123124. The iNO could be considered when patients appear to be at great risk of imminent death from hypoxemia despite all other treatments. In meta-analyses, the use of iNO showed insignificant beneficial effects on the duration of mechanical ventilation and length of stay in the ICU compared to the control group123124. As a result of meta-analyses, the improved oxygenation (PaO2/FIO2) was observed in the first 24 hours123125126. Although iNO is supposed to improve oxy-genation by reducing ventilation-perfusion mismatch at first, it will induce vasodilatation of poorly ventilated areas, increasing ventilation-perfusion mismatch127. Improved oxygenation is not associated with increased survival rate because the temporary improvement of oxygenation does not indicate improved lung function, reduction of lung injury, or resolution of the underlying cause of ARDS including coexisting multi-organ damage128.
With the use of iNO, the difference of pulmonary arterial pressure was initially significant at the first day but no longer present on day 2 to 4123. The use of iNO increased the risk of renal injury among adult patients with ARDS123129. Nitric oxide (NO) is known as an important regulator renal vascular tone and a modulator of glomerular function. Therefore, the changes in NO production could cause acute renal injury by altering the function of mitochondria, various enzymes, and DNA. Despite insufficient randomized controlled trials or meta-analyses, the combination therapies with prone positioning or HFOV may help in selected groups of patients or as a salvage therapy because they can enhance the effect of iNO than monotherapy alone128.
- We recommend low tidal volume ventilation can be applied in patients who require mechanical ventilation for diseases other than ARDS (grade 1B). To lower the incidence of pulmonary complications including ARDS in intraoperative patients, LPV strategy may be applied during the operation (grade 2B).
In mechanically ventilated patients without ARDS, the application of low tidal volume can decrease the incidence of ARDS130. In the only randomized control study, the occurrence of ARDS increased in the patient group with a tidal volume of 10 mL/kg rather than the group with tidal volume of 6 mL/kg (predicted body weight) among ICU patients requiring mechanical ventilation for 72 hours or more (RR, 5.1; 95% CI, 1.2–22.6; p=0.01)130. Moreover, the patient group with high tidal volume had an increased incidence of ARDS, and this study was terminated earlier. The studies on the prevention of ARDS are mostly observational cohort studies131132133134135, and meta-analysis is difficult to be conducted because of heterogeneity between studies. According to a systematic review conducted recently, however, in one randomized control study and most observational studies, the relationship between high tidal volume and the occurrence of ARDS136 was suggested. However, several observational studies showed that fluid imbalance135137 and transfusion131134135137138139 may also contribute to the occurrence of ARDS.
In patients requiring endotracheal intubation for general anesthesia and mechanical ventilation during surgery, the application of low tidal volume can lower postoperative pulmonary complications140141. The studies on mechanical ventilation during operation are limited in number, and the studies conducted so far are the results of observational studies142143 which dealt with the change in inflammatory cytokines considered to mediate the occurrence of ARDS rather than directly identifying the ARDS144145146. Both observational cohort studies which were conducted in patients without acute lung injury under non-thoracic surgery142 and a case-control study conducted in patients under various surgeries including lung surgery143 did not reveal the relationship between tidal volume and ARDS. However, the difference of tidal volume between patient groups might not be wide enough to make an ARDS. The tidal volume as the use of high tidal volume may be reduced because those studies were conducted after the study that the use of low tidal volume brought the reduction of mortality in ARDS. Also, there is an opinion that the use of PEEP was not generalized in the group applied by low tidal volume, so the occurrence of atelectasis could not be prevented142147. However, in a recent study148, the application of high PEEP increased the occurrence of hypotension during surgery, compared to low PEEP. Therefore, it is important to apply appropriate PEEP during surgery, but it is difficult to suggest its accurate levels. In the observational cohort study conducted in 1,091 patients who received a pneumonectomy within a 10-year period, the reduction of the occurrence of ARDS was found when a LPV strategy was performed during one-lung ventilation (adjusted OR, 0.34; 95% CI, 0.23–0.75; p=0.0002)141. There was a randomized control study conducted in patients who received a pneumonectomy, which showed both PaO2/FiO2 <300 mm Hg or the occurrence of new lung lesions were decreased in the group of lung protective strategy whereas the occurrence of ARDS did not show a statistical difference because of its small number149. A recent randomized control study140 conducted in patients who received abdominal surgery showed a similar result; the LPV strategy including low tidal volume and moderate PEEP lowered the occurrence of respiratory complications for 7 days after the abdominal operation, compared to non-lung protective strategy (adjusted RR, 0.49; 95% CI, 0.32–0.74; p<0.001). However, both the groups had very small number of occurrence of ARDS, and there was no statistical significance between the two groups (adjusted RR, 0.21; 95% CI, 0.02–1.71; p=0.14). In this study, the fact that pneumonia and sepsis occurrence, and the main risk factors for ARDS, were decreased in the LPV strategy group suggests the possibility that the use of LPV strategy during the operation under general anesthesia can reduce the occurrence of ARDS.
- We recommend light sedation should be conducted in critically ill patients who receive mechanical ventilation including ARDS (grade 1B).
- We suggest pain should regularly be evaluated in critically ill patients who receive mechanical ventilation in ICU.
- It is required to have a proper prevention for the occurrence of delirium caused by the absence of appropriate analgesia and sedation or other physical diseases.
Light sedation should be conducted in the mechanically ventilated patients including ARDS (1B). It is known that waking patients up every day or using light sedation will have clinically better results150151. As a method to evaluate sedation, it is recommended to use Richmond Agitation-Sedation Scale (RASS) or Sedation-Agitation Scale (SAS)152153. A variety of sedatives has been used to induce adequate sedation in patients. Recently, because using non-benzodiazepine sedatives (propofol and dexmedetomidine) is known to cause better clinical outcomes than using benzodiazepines (midazolam and diazepam) in mechanically ventilated patients including ARDS, it is recommended to conduct sedation with non-benzodiazepine drugs (2B)154.
It is recommended to evaluate the pain during ICU admission regularly for mechanically ventilated patients including ARDS155. Those who can communicate without endotracheal intubation can directly describe their pain by conducting visual analog scale or numeric rating scale, etc. However, patients who cannot directly express their pain because of endotracheal intubation or unconsciousness should evaluate the pain through behavioral pain scale156. It is recommended to use the intravenous infusion of narcotic analgesics as the primary treatment for pain control in ICU157. Fentanyl, hydromorphone, morphine, remifentanil, and methadone can be used as narcotic analgesics, and they should be applied to each patient individually according to pharmacokinetic and pharmacodynamic properties of each drug. Currently, methadone is not available in South Korea. The use of meperidine should be avoided because of risks including neurologic toxicity158. Non-narcotic analgesics (IV acetaminophen, a cyclooxygenase inhibitor, and ketamine) can be administered concomitantly to reduce side effects by narcotic analgesics. For neuropathic pain, the drugs including gabapentin or carbamazepine should be orally administered in combination with narcotic analgesics. In addition to IV analgesics administration, thoracic epidural analgesia is also effective for rib fracture or abdominal aortic aneurysm surgery154.
Since the pathophysiology and definitive treatment of delirium are not evaluated enough, further studies are still needed. According to the studies published so far, however, risk factors that may cause the occurrence of delirium include the seriousness of disease at ICU admission, history of alcohol use, and excessive physical binding in ICU159. Delirium is closely related to increase of various complications such as increased length of stay in ICU or hospital admission, and increased mortality as well as deterioration of cognitive functions159160. Therefore, it is recommended to evaluate the occurrence of delirium regularly. Confusion Assessment Methods for the ICU (CAM-ICU) or Intensive Care Delirium Screening Checklist (ICDSC) can be the most reliable evaluation methods to evaluate delirium in ICU161162. In delirium, prevention rather than treatment is more important. For the non-pharmacological method, early rehabilitation treatment such as early ambulation is recommended163. If delirium occurs, conservative treatment should be conducted, and excessive use of sedative should be avoided. There is no clear evidence on atypical antipsychotics such as haloperidol. The prevention and treatment of sedation, analgesia, and delirium in ICU cannot make individually, but should be connected each other. Also, to give adequate analgesics and sedatives to all mechanically ventilated patients including ARDS, a variety of multidisciplinary approaches and studies are required.
- We suggest early tracheostomy in patients who receive mechanical ventilation can be performed only in limited cases (grade 2A).
- Early tracheostomy may decrease the hospital length of stay in limited patients and the use of sedative drugs.
- Early tracheostomy may not lower ICU mortality and the incidence of ventilator-associated pneumonia (VAP), or shorten the duration of mechanical ventilation.
The definition of early tracheostomy varies among studies. Generally, the operation conducted before 7–10 days from intra-tracheal intubation can be defined as "early tracheostomy." Otherwise, "late" or "delayed" tracheostomy means that the operation was performed after 10 days of intubation. Three randomized control studies164165166, two meta-analyses167168, and one review article169 were examined. According to the results of recently published large-scale randomized control studies, all studies by Terragni et al.164, Trouillet et al.165, and Young et al.166 which were large-scale randomized control studies, did not show a mortality reduction in the early tracheostomy group. Meta-analyses studies by Wang et al.167 and Huang et al.168 did not also show an effect on short- and long-term mortality. The randomized control study by Terragni et al.164, revealed the number of ventilator-free days was significantly greater in the early tracheostomy group (11 days vs. 6 days, p=0.02), but the other randomized control studies and meta-analyses could not prove the statistical significance. All three randomized control studies and two meta-analyses164165166167168 showed that early tracheostomy did not shorten ICU length of stay and hospital length of stay. Only Terragni et al.164 showed that the number of ICU discharges was significantly higher in the early tracheostomy group (48% vs. 39%, p=0.03), but the duration of hospital stay was not different. However, the patients with a chronic pulmonary disorder or respiratory tract infection were all excluded in that study, and those were only a small proportion of mechanically ventilated patients. Thus, interpretation of the result requires special caution in clinical practice.
In the study by Terragni et al.164, the early tracheostomy group showed a lower incidence of VAP, but the study could not prove the statistical significance (14% vs. 21%, p=0.07). In meta-analyses, early tracheostomy had no effect on the reduction VAP incidence. In the study by Trouillet et al.165, reported early tracheostomy was associated with less need for intravenous sedation; the use of midazolam (mean difference, –0.31 mg/kg/day; 95% CI, –0.53 to –0.09 mg/kg/day), propofol (mean difference, –2.87 mg/kg/day; 95% CI, –4.76 to –0.98 mg/kg/day), and sufentanil (mean difference, –0.48 µg/kg/day; 95% CI, –0.77 to –0.19 µg/kg/day). Young et al.166 proved days demanding any sedative was significantly lower in the 30-day survivor subgroup analysis (5 days vs. 8 days; median difference, 2.4; 95% CI, 1.6–3.6 days; p<0.001).
In the recent meta-analyses and large-scale randomized control studies, early tracheostomy could not lower the mortality and incidence of VAP. Also, early tracheostomy did not significantly affect the duration of ICU stay and hospital stay. However, because these studies have a limitation on the subjects, and the results cannot be uniformly applied to all critical patients. Generally, tracheostomy should be considered in the following cases: (1) patients at risk of airway obstruction, (2) patients with recurrent weaning failure, (3) patients who need airway hygiene or toileting due to a bed-ridden or prolonged unconscious status. Thus, the optimal time for performing a tracheostomy should be determined after considering the clinical situation of patients, preference of patients, bronchial secretions, causes of respiratory failure, benefits or disadvantages of tracheostomy, and others. The basis for performing an early tracheostomy in patients who receive mechanical ventilation has not yet been established.
The research network initiated by the National Institutes of Health in the United States was recently dispersed while left decades of therapeutic trials for ARDS. Nevertheless, most of their results were negative, recent positive clinical trials of neuromuscular blockage and prone positioning suggested hopeful directions to make an advance in this uncontrollable clinical syndrome, in addition to the traditional lung protective strategy including low tidal volume ventilation, high PEEP, or recruitment maneuver. Moreover, ECMO, a revisited technique described more than decades ago, might be a still optional choice, however, systemic steroids and iNO would be less or no effective treatment based on up-to-date evidence. Besides ARDS, low tidal volume ventilation and light sedation should be considered in most of the patients who receive mechanical ventilation in the ICU and early tracheostomy could also be attempted in limited patients. Undoubtedly, over the next decade, ambitious research like the "ECMO to rescue Lung injury in severe ARDS (EOLIA)" trial would be conducted, and their results will contribute to revising this brand-new clinical practice guideline for mechanically ventilated patients with or without ARDS.
1. We recommend low tidal volume ventilation can be applied to patients with ARDS to reduce mortality (grade 1A).
The tidal volume should be maintained less than 6 mL/kg of predicted body weight in patients with ARDS.
The plateau pressure should be maintained less than 30 cmH2O in patients with ARDS.
2. We suggest high PEEP can be applied to patients with ARDS, who have PaO2/FIO2 ≤200 mm Hg to reduce mortality (grade 2B).
The application of high PEEP does not increase the risk of barotrauma.
If high PEEP is applied, PaO2/FIO2 at day 1 and three can be improved compared to the application of low PEEP group.
3. We recommend prone position can be applied to patients with moderate or above ARDS to reduce mortality if it is not contraindicated (grade 1B).
Prone position should be applied when there is no improvement of oxygenation at early stage of mechanical ventilation.
Prone position is recommended at least for 10 hours.
Lung protective strategy should also be applied during prone positioning.
4. We suggest ECMO as a rescue therapy in patients with ARDS without improvement of hypoxia by lung protective strategy (grade 2C).
5. We suggest recruitment maneuver can be applied to patients with ARDS to reduce mortality (grade 2B).
Recruitment maneuver has an effect on improving hypoxia, without increasing the risk of barotrauma.
6. The use of systemic steroids cannot reduce mortality in patients with ARDS (grade 2B).
In the case of a low dose of systemic steroid is used in the early stage, it may improve hypoxemia and reduce the period of mechanical ventilation, the length of ICU stay, and mortality.
7. We suggest neuromuscular blockade for 48 hours after starting mechanical ventilation in patients with ARDS (grade 2B).
The use of neuromuscular blockage in patients with ARDS has an effect on improvement of hypoxemia for first 48 hours.
The use of neuromuscular blockage can reduce barotrauma such as pneumothorax in patients with ARDS.
8. The use of HFOV should not be recommended as a standard treatment method in adult patients with ARDS (grade 1B).
HFOV does not improve survival in patients with ARDS.
HFOV may cause side effects such as barotrauma or low blood pressure.
9. The use of iNO should not be recommended as a standard treatment method in adult and child patients with ARDS (grade 1A).
The use of iNO in patients with ARDS may increase the risk of renal injury in adults.
10. We recommend low tidal volume ventilation can be applied in patients who require mechanical ventilation for diseases other than ARDS (grade 1B). To lower the incidence of pulmonary complications including ARDS in intraoperative patients, lung protective ventilation strategy may be applied during the operation (grade 2B).
11. We recommend light sedation should be conducted in critically ill patients who receive mechanical ventilation including ARDS (grade 1B).
We suggest pain should regularly be evaluated in critically ill patients who receive mechanical ventilation in ICU.
It is required to have a proper prevention for the occurrence of delirium caused by the absence of appropriate analgesia and sedation or other physical diseases.
12. We suggest early tracheostomy in patients who receive mechanical ventilation can be performed only in limited cases (grade 2A).
Early tracheostomy may decrease the hospital length of stay in limited patients.
Early tracheostomy may decrease the use of sedative drugs.
Early tracheostomy may not lower ICU mortality and the incidence of ventilator-associated pneumonia, or shorten the duration of mechanical ventilation.
Acknowledgements
We are appreciated of Seok Chan Kim, Sung-Won Na, Jong-Seo Yoon, Sang-Min Lee, Sang Hyun Lim, Sang-Bum Hong (from the Korean Society of Critical Care Medicine), Jae Yeol Kim, Moo Suk Park, Jang Won Sohn, Ki Man Lee, Heung-Bum Lee, Jae Wha Cho (from the Korean Academy of Tuberculosis and Respiratory Diseases) for devoted external review and thanks to Kyung Hwa Seo for supporting statistical analyses.
References
1. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967; 2:319–323. PMID: 4143721.

2. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000; 342:1334–1349. PMID: 10793167.

3. ARDS Definition, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307:2526–2533. PMID: 22797452.
4. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994; 9:72–81. PMID: 8199655.
5. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005; 353:1685–1693. PMID: 16236739.

6. Kim JH, Hong SK, Kim KC, Lee MG, Lee KM, Jung SS, et al. Influence of full-time intensivist and the nurse-to-patient ratio on the implementation of severe sepsis bundles in Korean intensive care units. J Crit Care. 2012; 27:414.e11–414.e21.

7. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406. PMID: 21208779.

8. Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest. 2006; 129:174–181. PMID: 16424429.
9. Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013; 66:719–725. PMID: 23312392.

10. Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013; 66:726–735. PMID: 23570745.

11. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995; 311:376–380. PMID: 7640549.
12. Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1985; 132:485–489. PMID: 4037521.

13. Bartlett RH, Morris AH, Fairley HB, Hirsch R, O'Connor N, Pontoppidan H. A prospective study of acute hypoxic respiratory failure. Chest. 1986; 89:684–689. PMID: 3698698.

15. Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998; 157(6 Pt 1):1721–1725. PMID: 9620897.
16. Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians. 1998; 110:482–488. PMID: 9824530.
17. International consensus conferences in intensive care medicine: ventilator-associated lung injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, The European Society of Intensive Care Medicine, and The Societe de Reanimation de Langue Francaise, and was approved by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 1999; 160:2118–2124. PMID: 10588637.
18. Imai Y, Slutsky AS. High-frequency oscillatory ventilation and ventilator-induced lung injury. Crit Care Med. 2005; 33(3 Suppl):S129–S134. PMID: 15753718.

19. Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007; 357:1113–1120. PMID: 17855672.

20. Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998; 338:347–354. PMID: 9449727.

21. Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998; 158:1831–1838. PMID: 9847275.
22. Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P Jr, Wiener CM, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999; 27:1492–1498. PMID: 10470755.

23. Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med. 1998; 338:355–361. PMID: 9449728.
24. Kairalla RA, Franca SA. Mechanical ventilation in acute respiratory distress syndrome: impact of the use of low frequency ventilation. Rev Assoc Med Bras (1992). 2000; 46:297. PMID: 11175550.
25. Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013; (2):CD003844. PMID: 23450544.

26. Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2007; (3):CD003844. PMID: 17636739.

27. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637. PMID: 23353941.

28. Villar J. The use of positive end-expiratory pressure in the management of the acute respiratory distress syndrome. Minerva Anestesiol. 2005; 71:265–272. PMID: 15886587.
29. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004; 351:327–336. PMID: 15269312.

30. Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008; 299:646–655. PMID: 18270353.
31. Coffey RL, Albert RK, Robertson HT. Mechanisms of physiological dead space response to PEEP after acute oleic acid lung injury. J Appl Physiol Respir Environ Exerc Physiol. 1983; 55:1550–1557. PMID: 6358162.

32. Dorinsky PM, Whitcomb ME. The effect of PEEP on cardiac output. Chest. 1983; 84:210–216. PMID: 6347545.

33. Eisner MD, Thompson BT, Schoenfeld D, Anzueto A, Matthay MA. Acute Respiratory Distress Syndrome Network. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002; 165:978–982. PMID: 11934725.

34. Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006; 34:1311–1318. PMID: 16557151.

35. Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009; 13:R22. PMID: 19239703.

36. Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008; 299:637–645. PMID: 18270352.
37. Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008; 359:2095–2104. PMID: 19001507.

38. Goligher EC, Kavanagh BP, Rubenfeld GD, Adhikari NK, Pinto R, Fan E, et al. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med. 2014; 190:70–76. PMID: 24919111.

39. Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo SG, et al. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2010; 181:578–586. PMID: 19910610.

40. Slutsky AS, Hudson LD. PEEP or no PEEP: lung recruitment may be the solution. N Engl J Med. 2006; 354:1839–1841. PMID: 16641401.
41. Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome: rationale, indications, and limits. Am J Respir Crit Care Med. 2013; 188:1286–1293. PMID: 24134414.

42. Guerin C, Baboi L, Richard JC. Mechanisms of the effects of prone positioning in acute respiratory distress syndrome. Intensive Care Med. 2014; 40:1634–1642. PMID: 25266133.

43. Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002; 20:1017–1028. PMID: 12412699.

44. Bryan AC. Conference on the scientific basis of respiratory therapy: pulmonary physiotherapy in the pediatric age group. Comments of a devil's advocate. Am Rev Respir Dis. 1974; 110(6 Pt 2):143–144. PMID: 4440945.
45. Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001; 345:568–573. PMID: 11529210.
46. Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med. 2008; 36:603–609. PMID: 18216609.

47. Kopterides P, Siempos II, Armaganidis A. Prone positioning in hypoxemic respiratory failure: meta-analysis of randomized controlled trials. J Crit Care. 2009; 24:89–100. PMID: 19272544.

48. Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010; 36:585–599. PMID: 20130832.

49. Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168. PMID: 23688302.

50. Lee JM, Bae W, Lee YJ, Cho YJ. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med. 2014; 42:1252–1262. PMID: 24368348.
51. Hu SL, He HL, Pan C, Liu AR, Liu SQ, Liu L, et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care. 2014; 18:R109. PMID: 24887034.

52. Sud S, Friedrich JO, Adhikari NK, Taccone P, Mancebo J, Polli F, et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2014; 186:E381–E390. PMID: 24863923.

53. Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009; 302:1977–1984. PMID: 19903918.
54. Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006; 173:1233–1239. PMID: 16556697.

55. Fernandez R, Trenchs X, Klamburg J, Castedo J, Serrano JM, Besso G, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 2008; 34:1487–1491. PMID: 18427774.

56. Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014; 40:332–341. PMID: 24435203.

57. Gattinoni L, Pesenti A. The concept of "baby lung". Intensive Care Med. 2005; 31:776–784. PMID: 15812622.

58. Galiatsou E, Kostanti E, Svarna E, Kitsakos A, Koulouras V, Efremidis SC, et al. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med. 2006; 174:187–197. PMID: 16645177.

59. Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004; 292:2379–2387. PMID: 15547166.
60. Kimmoun A, Guerci P, Bridey C, Ducrocq N, Vanhuyse F, Levy B. Prone positioning use to hasten veno-venous ECMO weaning in ARDS. Intensive Care Med. 2013; 39:1877–1879. PMID: 23835725.

61. Guervilly C, Hraiech S, Gariboldi V, Xeridat F, Dizier S, Toesca R, et al. Prone positioning during veno-venous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome in adults. Minerva Anestesiol. 2014; 80:307–313. PMID: 24257150.
62. Munshi L, Fan E, Del Sorbo L. Prone position during ECMO: a turn of events? Minerva Anestesiol. 2014; 80:281–283. PMID: 24326973.
63. Kipping V, Weber-Carstens S, Lojewski C, Feldmann P, Rydlewski A, Boemke W, et al. Prone position during ECMO is safe and improves oxygenation. Int J Artif Organs. 2013; 36:821–832. PMID: 24338657.

64. Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972; 286:629–634. PMID: 5060491.
65. Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA. 1979; 242:2193–2196. PMID: 490805.

66. Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994; 149(2 Pt 1):295–305. PMID: 8306022.

67. Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011; 306:1659–1668. PMID: 21976615.

68. Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013; 187:276–285. PMID: 23155145.
69. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009; 374:1351–1363. PMID: 19762075.

70. Ventetuolo CE, Muratore CS. Extracorporeal life support in critically ill adults. Am J Respir Crit Care Med. 2014; 190:497–508. PMID: 25046529.

71. Zampieri FG, Mendes PV, Ranzani OT, Taniguchi LU, Pontes Azevedo LC, Vieira Costa EL, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care. 2013; 28:998–1005. PMID: 23954453.

72. Lee JJ, Hwang SM, Ko JH, Kim HS, Hong KS, Choi HH, et al. Efficacy of veno-venous extracorporeal membrane oxygenation in severe acute respiratory failure. Yonsei Med J. 2015; 56:212–219. PMID: 25510767.

73. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011; 365:1905–1914. PMID: 22087681.

74. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014; 189:1374–1382. PMID: 24693864.

75. Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, et al. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999; 159:872–880. PMID: 10051265.

76. Lim CM, Koh Y, Park W, Chin JY, Shim TS, Lee SD, et al. Mechanistic scheme and effect of "extended sigh" as a recruitment maneuver in patients with acute respiratory distress syndrome: a preliminary study. Crit Care Med. 2001; 29:1255–1260. PMID: 11395617.

77. Hodgson C, Keating JL, Holland AE, Davies AR, Smirneos L, Bradley SJ, et al. Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database Syst Rev. 2009; (2):CD006667. PMID: 19370647.

78. Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, et al. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med. 2008; 178:1156–1163. PMID: 18776154.
79. Suzumura EA, Figueiro M, Normilio-Silva K, Laranjeira L, Oliveira C, Buehler AM, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and metaanalysis. Intensive Care Med. 2014; 40:1227–1240. PMID: 25097070.

80. Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, et al. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002; 96:795–802. PMID: 11964585.

81. Foti G, Cereda M, Sparacino ME, De Marchi L, Villa F, Pesenti A. Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Med. 2000; 26:501–507. PMID: 10923722.

82. Pelosi P, Caironi P, Gattinoni L. Pulmonary and extrapulmonary forms of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2001; 22:259–268. PMID: 16088678.

83. Sibbald WJ, Anderson RR, Reid B, Holliday RL, Driedger AA. Alveolo-capillary permeability in human septic ARDS. Effect of high-dose corticosteroid therapy. Chest. 1981; 79:133–142. PMID: 7460641.
84. Rocco PR, Souza AB, Faffe DS, Passaro CP, Santos FB, Negri EM, et al. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med. 2003; 168:677–684. PMID: 12842856.

85. Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998; 158(5 Pt 1):1432–1441. PMID: 9817690.

86. Weigelt JA, Norcross JF, Borman KR, Snyder WH 3rd. Early steroid therapy for respiratory failure. Arch Surg. 1985; 120:536–540. PMID: 3885915.
87. Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987; 317:1565–1570. PMID: 3317054.

88. Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987; 92:1032–1036. PMID: 3315478.

89. Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998; 280:159–165. PMID: 9669790.

90. Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006; 354:1671–1684. PMID: 16625008.

91. Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007; 131:954–963. PMID: 17426195.
92. Annane D, Sebille V, Bellissant E. Ger-Inf-05 Study Group. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med. 2006; 34:22–30. PMID: 16374152.

93. Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med. 2004; 3:307–328. PMID: 15606221.
94. Agarwal R, Nath A, Aggarwal AN, Gupta D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology. 2007; 12:585–590. PMID: 17587427.

95. Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008; 336:1006–1009. PMID: 18434379.

96. Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009; 37:1594–1603. PMID: 19325471.

97. Lamontagne F, Briel M, Guyatt GH, Cook DJ, Bhatnagar N, Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010; 25:420–435. PMID: 19896324.

98. Ruan SY, Lin HH, Huang CT, Kuo PH, Wu HD, Yu CJ. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2014; 18:R63. PMID: 24708846.

99. Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008; 34:61–69. PMID: 18000649.

100. Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005; 171:242–248. PMID: 15557131.
101. Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006; 34:2749–2757. PMID: 16932229.

102. Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004; 32:113–119. PMID: 14707568.

103. Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363:1107–1116. PMID: 20843245.

104. Steingrub JS, Lagu T, Rothberg MB, Nathanson BH, Raghunathan K, Lindenauer PK. Treatment with neuromuscular blocking agents and the risk of in-hospital mortality among mechanically ventilated patients with severe sepsis. Crit Care Med. 2014; 42:90–96. PMID: 23982029.

105. Goddard SL, Fan E. "Only few find the way, some don't recognize it when they do...": can we "observe" causality? Crit Care Med. 2014; 42:208–209. PMID: 24346530.

106. Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013; 17:R43. PMID: 23497608.

107. Kondili E, Prinianakis G, Hoeing S, Chatzakis G, Georgopoulos D. Low flow inflation pressure-time curve in patients with acute respiratory distress syndrome. Intensive Care Med. 2000; 26:1756–1763. PMID: 11271082.

108. Herridge MS. Building consensus on ICU-acquired weakness. Intensive Care Med. 2009; 35:1–3. PMID: 18946660.

109. Leatherman JW, Fluegel WL, David WS, Davies SF, Iber C. Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med. 1996; 153:1686–1690. PMID: 8630621.

110. Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001; 27:1288–1296. PMID: 11511941.

111. Elliot JM, Bion JF. The use of neuromuscular blocking drugs in intensive care practice. Acta Anaesthesiol Scand Suppl. 1995; 106:70–82. PMID: 8533552.

113. Huang CT, Lin HH, Ruan SY, Lee MS, Tsai YJ, Yu CJ. Efficacy and adverse events of high-frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome: a meta-analysis. Crit Care. 2014; 18:R102. PMID: 24886674.

114. Maitra S, Bhattacharjee S, Khanna P, Baidya DK. High-frequency ventilation does not provide mortality benefit in comparison with conventional lung-protective ventilation in acute respiratory distress syndrome: a meta-analysis of the randomized controlled trials. Anesthesiology. 2015; 122:841–851. PMID: 24830508.
115. Bollen CW, van Well GT, Sherry T, Beale RJ, Shah S, Findlay G, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care. 2005; 9:R430–R439. PMID: 16137357.
116. Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013; 368:795–805. PMID: 23339639.

117. Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002; 166:801–808. PMID: 12231488.
118. Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013; 368:806–813. PMID: 23339638.

119. Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005; 128:525–532. PMID: 16100134.

120. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000; 342:1301–1308. PMID: 10793162.
121. Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, et al. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2012; 40:1539–1545. PMID: 22511135.

122. Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002; 28:406–413. PMID: 11967593.

123. Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg. 2011; 112:1411–1421. PMID: 21372277.
124. Adhikari NK, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014; 42:404–412. PMID: 24132038.
125. Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM, et al. Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med. 2003; 167:1008–1015. PMID: 12663340.
126. Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004; 291:1603–1609. PMID: 15069048.
127. Kass LJ, Apkon M. Inhaled nitric oxide in the treatment of hypoxemic respiratory failure. Curr Opin Pediatr. 1998; 10:284–290. PMID: 9716891.

128. Fan E, Mehta S. High-frequency oscillatory ventilation and adjunctive therapies: inhaled nitric oxide and prone positioning. Crit Care Med. 2005; 33(3 Suppl):S182–S187. PMID: 15753726.

129. Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007; 334:779. PMID: 17383982.

130. Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010; 14:R1. PMID: 20055989.

131. Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004; 32:1817–1824. PMID: 15343007.

132. Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005; 31:922–926. PMID: 15856172.

133. Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007; 35:1815–1820. PMID: 17568331.

134. Yilmaz M, Keegan MT, Iscimen R, Afessa B, Buck CF, Hubmayr RD, et al. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007; 35:1660–1666. PMID: 17507824.

135. Jia X, Malhotra A, Saeed M, Mark RG, Talmor D. Risk factors for ARDS in patients receiving mechanical ventilation for > 48 h. Chest. 2008; 133:853–861. PMID: 18263691.
136. Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit Care. 2013; 17:R11. PMID: 23331507.

137. Plurad D, Martin M, Green D, Salim A, Inaba K, Belzberg H, et al. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: the potential role of lung protective ventilation and conservative transfusion practice. J Trauma. 2007; 63:1–7. PMID: 17622861.

138. Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008; 36:1518–1522. PMID: 18434908.

139. Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006; 34:196–202. PMID: 16374174.

140. Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013; 369:428–437. PMID: 23902482.

141. Licker M, Diaper J, Villiger Y, Spiliopoulos A, Licker V, Robert J, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care. 2009; 13:R41. PMID: 19317902.

142. Hughes CG, Weavind L, Banerjee A, Mercaldo ND, Schildcrout JS, Pandharipande PP. Intraoperative risk factors for acute respiratory distress syndrome in critically ill patients. Anesth Analg. 2010; 111:464–467. PMID: 20418537.

143. Fernandez-Perez ER, Sprung J, Afessa B, Warner DO, Vachon CM, Schroeder DR, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009; 64:121–127. PMID: 18988659.

144. Wrigge H, Uhlig U, Zinserling J, Behrends-Callsen E, Ottersbach G, Fischer M, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg. 2004; 98:775–781. PMID: 14980936.

145. Choi G, Wolthuis EK, Bresser P, Levi M, van der Poll T, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology. 2006; 105:689–695. PMID: 17006066.

146. Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008; 108:46–54. PMID: 18156881.

147. Hemmes SN, Serpa Neto A, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol. 2013; 26:126–133. PMID: 23385321.
148. PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014; 384:495–503. PMID: 24894577.
149. Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, Kim MJ, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery? A randomized controlled trial. Chest. 2011; 139:530–537. PMID: 20829341.
150. Martin J, Heymann A, Basell K, Baron R, Biniek R, Burkle H, et al. Evidence and consensus-based German guidelines for the management of analgesia, sedation and delirium in intensive care: short version. Ger Med Sci. 2010; 8:Doc02. PMID: 20200655.
151. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342:1471–1477. PMID: 10816184.

152. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–1344. PMID: 12421743.
153. Ryder-Lewis MC, Nelson KM. Reliability of the Sedation-Agitation Scale between nurses and doctors. Intensive Crit Care Nurs. 2008; 24:211–217. PMID: 18206372.

154. Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306. PMID: 23269131.

155. Kastrup M, von Dossow V, Seeling M, Ahlborn R, Tamarkin A, Conroy P, et al. Key performance indicators in intensive care medicine: a retrospective matched cohort study. J Int Med Res. 2009; 37:1267–1284. PMID: 19930832.

156. Aissaoui Y, Zeggwagh AA, Zekraoui A, Abidi K, Abouqal R. Validation of a behavioral pain scale in critically ill, sedated, and mechanically ventilated patients. Anesth Analg. 2005; 101:1470–1476. PMID: 16244013.
157. Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007; 106:687–695. PMID: 17413906.
158. Erstad BL, Puntillo K, Gilbert HC, Grap MJ, Li D, Medina J, et al. Pain management principles in the critically ill. Chest. 2009; 135:1075–1086. PMID: 19349403.

159. Mehta S, Cook D, Devlin JW, Skrobik Y, Meade M, Fergusson D, et al. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med. 2015; 43:557–566. PMID: 25493968.

160. Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010; 38:1513–1520. PMID: 20473145.

161. Plaschke K, von Haken R, Scholz M, Engelhardt R, Brobeil A, Martin E, et al. Comparison of the confusion assessment method for the intensive care unit (CAM-ICU) with the Intensive Care Delirium Screening Checklist (ICDSC) for delirium in critical care patients gives high agreement rate(s). Intensive Care Med. 2008; 34:431–436. PMID: 17994221.

162. Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012; 16:R115. PMID: 22759376.

163. Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009; 373:1874–1882. PMID: 19446324.

164. Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010; 303:1483–1489. PMID: 20407057.
165. Trouillet JL, Luyt CE, Guiguet M, Ouattara A, Vaissier E, Makri R, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial. Ann Intern Med. 2011; 154:373–383. PMID: 21403073.
166. Young D, Harrison DA, Cuthbertson BH, Rowan K. TracMan Collaborators. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013; 309:2121–2129. PMID: 23695482.
167. Wang F, Wu Y, Bo L, Lou J, Zhu J, Chen F, et al. The timing of tracheotomy in critically ill patients undergoing mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011; 140:1456–1465. PMID: 21940770.
168. Huang H, Li Y, Ariani F, Chen X, Lin J. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One. 2014; 9:e92981. PMID: 24667875.

169. Gomes Silva BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2012; (3):CD007271. PMID: 22419322.

Table 1
Summary of recommendations and level of evidence for mechanically ventilated patients with or without acute respiratory distress syndrome
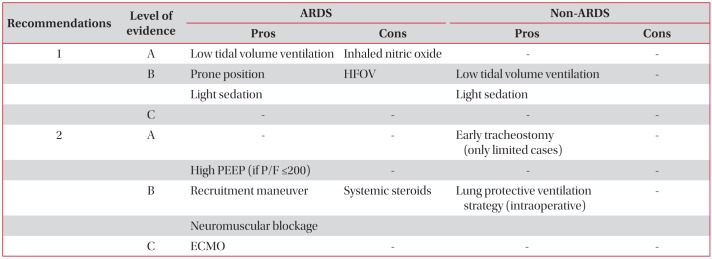




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download