Abstract
Several chemotherapeutic agents are known to develop pulmonary toxicities in cancer patients, although the frequency of incidence varies. Cyclophosphamide is a commonly encountered agent that is toxic to the lung. Additionally, granulocyte colony-stimulating factor (G-CSF) being used for the recovery from neutropenia can exacerbate lung injury. However, most of the patients reported previously that the drug-induced interstitial pneumonitis were developed after three to four cycles of chemotherapy. Hereby, we report a case of peripheral T cell lymphoma which rapidly developed a fatal interstitial pneumonitis after the first cycle of combined chemotherapy with cyclophosphamide, adriamycin, vincristine, prednisolone, and etoposide and the patient had also treated with G-CSF during neutropenic period.
Patients with hematological malignancies can develop to various acute pulmonary diseases during the course of chemotherapy, and interstitial pneumonitis induced by chemotherapeutic agents is commonly seen as a pulmonary complication1,2. In addition, the use of granulocyte colony-stimulating factor (G-CSF) during the treatment of chemotherapy-induced neutropenia enhanced the lung injury2-5. However, in most reported cases, the patients developed chemotherapeutic drug-induced interstitial lung disease after several cycles of chemotherapy1.
Here, we report a rare case of peripheral T cell lymphoma, not otherwise specified (PTCL, NOS), with rapidly deteriorating fatal interstitial lung disease that developed after the first cycle of cyclophosphamide, adriamycin, vincristine, and prednisolone (CHOP) and etoposide combination chemotherapy along with G-CSF treatment.
A 69-year-old man was admitted to the hospital presenting with multiple palpable masses in the neck and groin area. He had hypertension and a 30-pack year smoking history. The Eastern Cooperative Oncology Group performance status was grade 2, and lactate dehydrogenase level was 824 IU/L (normal, 218-472 IU/L) at diagnosis. A lymph node biopsy was performed in the right groin, and the patient was diagnosed with PTCL, NOS. Further, neck, chest, and abdominal computed tomography (CT), positron emission tomography/computed tomography, and bone marrow examination were conducted for the staging work-up. Lymphomatous involvements were found in the tonsil, spleen, left anterior chest wall, ileocecal area, and multiple lymph nodes on both sides of the diaphragm (Figure 1). However, no involvement in the bone marrow was noted. The initial chest CT scan showed multiple lymphadenopathies and, incidentally, diffuse centrilobular emphysema (Figure 2). Finally, the patient was diagnosed with Ann Arbor stage IV PTCL, NOS and he was considered to be at high risk according to the international prognostic index. He received the first cycle of CHOP with etoposide combination chemotherapy (750 mg/m2 cyclophosphamide i.v. on day 1 [D1], 1.4 mg/m2 vincristine i.v. on D1, 50 mg/m2 doxorubicin i.v. on D1, 60 mg/m2 prednisolone p.o. on D1-5, and etoposide 100 mg/m2 i.v. on D1). Eight days after the chemotherapy, he developed a neutropenia, and, then, lenograstim (5 µg/kg, i.v.) was administered for 7 consecutive days. The second cycle of chemotherapy was delayed for 1 week because he had developed a moderate degree of small bowel bleeding while recovering from the neutropenia; the bleeding was controlled by supportive care only.
After 30 days of the first cycle of combination chemotherapy, the patient was hospitalized for the second cycle of CHOP with etoposide chemotherapy. Unfortunately, he complained of exertional dyspnea and shortness of breath without fever or purulent sputum. Crackles were noted in both lower lung fields on chest auscultation. The initial laboratory examination revealed a white blood cell count of 12,900/µL, hemoglobin level of 12.9 g/dL, and platelet count of 328,000/µL. The level of C-reactive protein was 14.31 mg/dL. Arterial blood gas analysis (ABGA) with 2 L/min oxygen administered via nasal prongs showed that the pH was 7.489, PaO2 75.9 mm Hg, PaCO2 28.4 mm Hg, bicarbonate level 21.1 mmol/L, and SaO2 96.3%, showing respiratory alkalosis. A chest X-ray showed diffuse ground glass appearances with reticulation in both lungs (Figure 3). A chest CT scan showed to the markedly improvements of previous multiple lymphomatous involvements in the thorax, but it also revealed that both lungs had a newly developed peripherally predominant diffuse ground glass appearance with reticulation (Figure 2).
For evaluation of associated pulmonary infection, the patient was performed the rapid influenza antigen immunoassays that can identify influenza A and B viral nucleoprotein antigens, sputum culture for the evaluation of bacterial pneumonia, acid-fast bacillus (AFB) smear, Mycobacterium tuberculosis polymerase chain reaction (PCR) test and Pneumocystis PCR in the sputum. Additionally, we checked the serum galactomannan assay for screening of aspergillosis infection before treatment of antibiotics. The influenza A or B antigen immunoassay, AFB stain, M. tuberculosis PCR test and Pneumocystis PCR were all negative and bacteria was not cultured in the respiratory specimen. The galactomannan assay showed the negative result (0.1 optical density index) and there was no objective evidence about pulmonary infection. Further diagnostic studies such as bronchoalveolar lavage (BAL) or lung biopsy were not performed because the symptom of dyspnea was deteriorated. Based on the clinical status and the radiological finding, the patient was diagnosed with drug-induced interstitial pneumonitis, and 125 mg/day prednisone was administered immediately with the empirical antibiotics such as tazobactam and clarithromycin. After the 15th day of prednisone therapy, a chest CT scan showed no changes, and a small amount of pneumomediastinum had newly developed (Figure 4). After the 32th day of prednisone treatment, the symptoms of dyspnea were aggravated up to grade 4 according to the Medical Research Council Dyspnea Scale, and a chest X-ray showed diffuse interstitial lung infiltration throughout the entire lung (Figure 2). At that time, we did not check the additional evaluations, such as Pneumocystis PCR or BAL study, because of the worsened his condition. ABGA showed that the patient was experiencing respiratory distress as his PaO2 was 59.6 mm Hg with 10 L/min oxygen supply via mask. Therefore, 375 mg/day prednisone was administered, but the patient died of respiratory failure on the 38th day after the treatment of prednisone.
The previously reported chemotherapeutic drugs that induce interstitial pneumonitis include bleomycin, methotrexate, cyclophosphamide, and busulfan2. In fact, the mechanisms by which bleomycin and cyclophosphamide induce pulmonary damages have been studied in detail6. These drugs induce the production of O2 radicals by neutrophils (sometimes in the presence of Cu2+ or Fe2+), which result in the lipid peroxidation and DNA damage7. It follows that interstitial pneumonitis induced by these drugs is exacerbated under conditions of high O2 pressure. Thus, O2 radicals produced by neutrophils possibly contribute to the development of drug-induced interstitial pneumonitis, particularly in lungs already damaged by certain chemotherapeutics8. G-CSF has been reported to stimulate superoxide production in neutrophils when administered with N-formylmethionyl-leucyl-phenylalanine or ionomycin in vitro9, G-CSF probably induces interstitial pneumonitis through neutrophil elastase production10. G-CSF is usually produced by fibroblasts, and it in turn enhances their proliferation11. Thus, these four mechanisms, namely, 1) superoxide production by cytotoxic drugs, 2) oxidant production by G-CSF, 3) elastase production by G-CSF, and 4) fibroblast proliferation by G-CSF, are potentially involved in the development of interstitial pneumonitis. In our case, chemotherapeutic agents, including cyclophosphamide, and G-CSF administration, can be considered the contributing factors in the development of interstitial pneumonitis.
The interval between the administration of chemotherapeutic agents and the onset of respiratory symptoms varies from weeks to years12. However, in many cases reported around the world, interstitial developments were observed in the third or subsequent chemotherapeutic cycles13,14. However, in the present case, the patient showed clinical signs of acute interstitial pneumonitis only after the first cycle of chemotherapy. He had a 30-pack year smoking history, and the initial chest CT scan had shown emphysematous changes. Previous studies established that pneumonitis associated with chemotherapy occurred significantly and more frequently in patients with preexisting interstitial lung disease15. Therefore, the cyclophosphamide and G-CSF may have accelerated the lung damage in this patient who had an existing lung disease.
This case has several limitations because we did not perform the lung biopsy for tissue confirmation and the BAL to identify the bacteriologic pathogen also was not checked for reason of deteriorated condition. However, the laboratory findings, the typical pulmonary fibrosis on the imaging study, and rapid clinical courses strongly indicated the diagnosis of drug-induced interstitial pneumonitis.
This patient illustrates the PTCL, NOS, with rapidly deteriorating, fatal interstitial lung disease that developed after the first cycle of chemotherapy along with G-CSF therapy. Modification of the G-CSF dose may be useful in preventing pneumotoxicity, and early application of a high-dose corticosteroid for patients with interstitial pneumonitis would be considered in addition to the discontinuation of G-CSF1. Therefore, clinicians should be alert to the signs and symptoms of lung injury during chemotherapy, especially in patients with preexisting interstitial lung disease.
Figures and Tables
Figure 1
Positron emission tomography scan showing disseminated lymphomatous involvements in the tonsils, spleen, left anterior chest wall, and multiple lymph nodes on both sides of the diaphragm.

Figure 2
Follow-up chest computed tomography scan during chemotherapy. (A) At diagnosis of peripheral T cell lymphoma, not otherwise specified, multiple lymph node enlargement and diffuse centrilobular emphysema were observed. (B) After the first cycle of chemotherapy with cyclophosphamide, adriamycin, vincristine, and prednisolone along with etoposide, the scan shows newly-developed diffused ground glass opacities with reticulation and peripheral predominance in both lungs.

Figure 3
(A) Chest X-ray scans at diagnosis of drug-induced interstitial pneumonitis shows diffused ground glass appearance with reticulation in both lung. (B) After 32th-day of prednisone treatment, chest X-ray scans show remarkable aggravation of diffused interstitial lung infiltration throughout the entire lung.
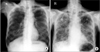
Figure 4
Chest computed tomography (CT) scan showing small amounts of newly appearing pneumomediastinum (black arrow), without evidence of aero-digestive tract injury (B) comparison with the follow-up chest CT on 30th days after the first cycle of chemotherapy with cyclophosphamide, adriamycin, vincristine, and prednisolone along with etoposide (A).

References
1. Nakase K, Tsuji K, Nagaya S, Tamaki S, Tanigawa M, Ikeda T, et al. Acute interstitial pneumonitis during chemotherapy for haematological malignancy. Eur J Cancer Care (Engl). 2005; 14:336–341.
2. Meadors M, Floyd J, Perry MC. Pulmonary toxicity of chemotherapy. Semin Oncol. 2006; 33:98–105.
3. Azoulay E, Attalah H, Yang K, Herigault S, Jouault H, Brun-Buisson C, et al. Exacerbation with granulocyte colony-stimulating factor of prior acute lung injury during neutropenia recovery in rats. Crit Care Med. 2003; 31:157–165.
4. Aggarwal A, Baker CS, Evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000; 15:895–901.
5. Wiedermann FJ, Mayr AJ, Hobisch-Hagen P, Fuchs D, Schobersberger W. Association of endogenous G-CSF with anti-inflammatory mediators in patients with acute respiratory distress syndrome. J Interferon Cytokine Res. 2003; 23:729–736.
6. Cooper JA Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: Cytotoxic drugs. Am Rev Respir Dis. 1986; 133:321–340.
7. Burger RM, Peisach J, Horwitz SB. Activated bleomycin: a transient complex of drug, iron, and oxygen that degrades DNA. J Biol Chem. 1981; 256:11636–11644.
8. Lindemann A, Herrmann F, Oster W, Haffner G, Meyenburg W, Souza LM, et al. Hematologic effects of recombinant human granulocyte colony-stimulating factor in patients with malignancy. Blood. 1989; 74:2644–2651.
9. Yuan L, Inoue S, Saito Y, Nakajima O. An evaluation of the effects of cytokines on intracellular oxidative production in normal neutrophils by flow cytometry. Exp Cell Res. 1993; 209:375–381.
10. Iijima S, Tsuji T, Sugita M, Yamada N, Satoh T, Kubo T. Leukocyte activity and occurrence of tissue injury by G-CSF. Nihon Sanka Fujinka Gakkai Zasshi. 1993; 45:213–219.
11. Mendoza JF, Caceres JR, Santiago E, Mora LM, Sanchez L, Corona TM, et al. Evidence that G-CSF is a fibroblast growth factor that induces granulocytes to increase phagocytosis and to present a mature morphology, and that macrophages secrete 45-kd molecules with these activities as well as with G-CSF-like activity. Exp Hematol. 1990; 18:903–910.
12. Brieva J. Cyclophosphamide-induced acute respiratory distress syndrome. Respirology. 2007; 12:769–773.
13. Katsuya H, Suzumiya J, Sasaki H, Ishitsuka K, Shibata T, Takamatsu Y, et al. Addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy has a high risk of developing interstitial pneumonia in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2009; 50:1818–1823.
14. Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, et al. Increased incidence of interstitial pneumonia by CHOP combined with rituximab. Int J Hematol. 2008; 87:393–397.
15. Togashi Y, Masago K, Handa T, Tanizawa K, Okuda C, Sakamori Y, et al. Prognostic significance of preexisting interstitial lung disease in Japanese patients with small-cell lung cancer. Clin Lung Cancer. 2012; 13:304–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download