Abstract
Background
This study was designed to analyze the efficacy of gefitinib as a second-line therapy, according to the clinical characteristics in Korean patients with non-small-cell lung cancer (NSCLC).
Methods
In this Phase IV observational study, we recruited patients, previously failed first-line chemotherapy, who had locally advanced or metastatic NSCLC, and who were found to be either epidermal growth factor receptor (EGFR) mutation-positive or satisfied 2 or more of the 3 characteristics: adenocarcinoma, female, and non-smoker. These patients were administered with gefitinib 250 mg/day, orally. The primary endpoints were to evaluate the objective response rate (ORR) and to determine the relationship of ORRs, depending on each patient's characteristics of modified intent-to-treat population.
Results
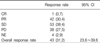
A total of 138 patients participated in this study. One subject achieved complete response, and 42 subjects achieved partial response (ORR, 31.2%). The subgroup analysis demonstrated that the ORR was significantly higher in patients with EGFR mutation-positive, compared to that of EGFR mutation-negative (45.8% vs. 14.0%, p=0.0004). In a secondary efficacy variable, the median progression-free survival (PFS) was 5.7 months (95% confidence interval, 3.9~8.4 months) and the 6-month PFS and overall survival were 49.6% and 87.9%, respectively. The most common reported adverse events were rash (34.4%), diarrhea (26.6%), pruritus (17.5%), and cough (15.6%).
Conclusion
Gefitinib was observed in anti-tumor activity with favorable tolerability profile as a second-line therapy in these selected patients. When looking at EGFR mutation status, EGFR mutation-positive showed strong association with gefitinib by greater response and prolonged PFS, compared with that of EGFR mutation-negative.
Lung cancer is the leading cause of cancer-related death worldwide. Non-small-cell lung cancer (NSCLC) accounts for the majority of all lung cancer cases. However, there is little known about the early pathogenesis of NSCLC, except smoking history. Recently, abnormal activation of epidermal growth factor receptor (EGFR) signaling is implicated in the growth of many solid tumors including NSCLC1.
Gefitinib is a potent and selective inhibitor of the EGFR tyrosine kinase, thus it hinders mitogenic and anti-apoptotic signals such as cell hyperplasia, growth metastasis, neovascularization, which work in the course of cancer progress. Gefitinib has shown anti-tumor activity in various solid tumors, most notable in NSCLC2-5.
In patients with NSCLC, the characteristics of adenocarcinoma, female, non-smoking, and Asian ethnicity have all been shown to increase the response of gefitinib6,7. As molecular predictive markers of outcome with gefitinib have been investigated, activating mutations of the EGFR have been shown to have a significant association with response to gefitinib8-10.
In a retrospective study, the patients who had received gefitinib with advanced NSCLC in Korea were participated. The result of the objective response rate (ORR) was 64.7% (11/17 patients) with EGFR mutation-positive and 13.7% (10/73 patients) with EGFR mutation-negative3.
Recently, the Phase III IRESSA Pan ASia Study (IPASS) compared gefitinib with carboplatin/paclitaxel as first-line treatment to non/light exsmokers who had lung adenocarcinoma in East Asia. In the study, progression-free survival (PFS) demonstrated significant and longer response in EGFR mutation-positive subgroup among the gefitinib group, and EGFR mutation-negative subgroup among the carboplatin/paclitaxel group11.
The Phase III IRESSA NSCLC Trial Evaluating Response and Survival against Taxotere (INTEREST) study has shown overall survival (OS) rate between gefitinib and docetaxel. The result was observed to be non-inferior with the patients who were unselect, previously treated with local advanced or metastatic NSCLC12.
Compared to above studies, this Phase IV, multicenter, non-randomized, open-label observational study (Second-Line IRESSA Phase IV observational study in NSCLC patient [SELINE]; NCT00608868) was conducted to analyze the efficacy of gefitinib as a second-line therapy according to the clinical characteristics in selected Korean patients with advanced NSCLC.
The study was approved by each Institutional Review Board (IRB) before initiation and was conducted in accordance with International Conference on Harmonisation (ICH) guidelines and applicable regulations. All patients signed written informed consent forms prior to participation of this study.
This study recruited advanced or metastatic NSCLC patients with histologic or cytologic-confirmed in Korea. Patients, who had progressive or recurrent disease with 19 to 80 years old following first-line chemotherapy, and who had either a positive EGFR mutation result or met 2 or more of the 3 characteristics: adenocarcinoma, female, and non-smoker, were participated in this study. Patients were also required to have a measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, a World Health Organization (WHO) performance status 0~1, and a life expectancy of at least 12 weeks.
Exclusion criteria included patients with any evidence of clinically active interstitial lung disease, malignancies, severe/uncontrolled systemic disease, treatment with a non-approved or investigational drug within 30 days prior to the study treatment, and those who have history of EGFR inhibitor treatment.
Before the start of treatment, all patients underwent the screening procedure, which included a physical examination, medical history, vital signs, WHO performance status, laboratory parameters, tumor assessment (RECIST), Quality of Life (QoL) assessment (by Functional Assessment of Cancer Therapy-Lung [FACT-L] Korean version 4), and EGFR mutation analysis. Determination of EGFR mutation was performed at a central laboratory (Isu Abxis Co., Ltd., 6th Yonsei University Medical Center, Seoul, Korea) or at 2 local laboratories (Konkuk University Hospital and Chonnam National University Hwasun Hospital). DNA was extracted from section of paraffin-embedded tumor (primary or recurred) tissue block, then was analyzed by using standard polymerase chain reaction-techniques and direct sequencing methods on exon 18, 19, and 21 of EGFR gene.
Patients visited every 4 weeks until the confirmation of disease progression and were performed the following assessments/procedures in every visit: physical examination, vital signs, WHO performance status, laboratory test, adverse events (AEs), concomitant medication, chest X-ray, and drug compliance. During the treatment period, tumor assessments by computed tomography scan and QoL by FACT-L were evaluated every 8 weeks. Once disease progression was confirmed, survival rate was follow-up every 12 weeks.
Gefitinib 250 mg (Iressa; AsatraZeneca, London, England) was administered orally once daily until the investigators gave confirmation of disease progression or of unacceptable toxicity or until patient refuse to participate in the study.
Response to treatment was assessed according to the RECIST criteria13. According to the RECIST criteria, ORR was defined as the proportion of subjects indicating partial response (PR) or complete response (CR) among the best responses assessed by the investigators. PFS was defined as the interval of the date of starting treatment and the objective disease progression or the absence of objective disease progression or death due to any cause. In addition, OS was defined as the interval of the date of starting treatment and the patient death due to any cause. Toxicity was classified in accordance with guideline of National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
The planned sample size was 219, the evaluable patients, considering drop-out rate of 10% wherein a response rate (P0) of 21.5% and (P1) of 30%. The establishment was based on Expanded Access Program with α of 0.05 (two-sided) and power of 0.8. Subjects who received at least one dose of gefitinib and had at least one tumor assessment were included in the modified intent-to-treat (mITT) analysis data set and the primary analysis was performed on the mITT set. The primary efficacy endpoint, ORR, was defined as the proportion of subjects showing PR or CR as the best response until final tumor assessment. Sub-analysis was done in subgroups that were defined according to EGFR mutation status, gender, smoking history, and tumor histology. All statistical analyses of ORR were performed using the chi-square test. Median survival time and 95% confidence interval (CI) of PFS or OS was calculated by using the Kaplan-Meier method and survival curve was presented and comparisons of PFS or OS were performed using the log-rank test. The improvement rate for QOL and for symptom improvement was defined as the change of point ≥6 in the summed FACT-L score from baseline and the change of point ≥2 in the summed lung cancer subscale (LCS) of FACT-L from baseline, respectively. Safety summaries were based on the safety set, which included all subjects who had received at least one dose of gefitinib and had at least one safety follow-up. Safety data included any AEs that occurred after the administration of study drug and any abnormalities in laboratory values at each visit. AE coding complied with MedDRA (version 12.0).
From January 2007 to July 2008, 156 subjects were enrolled from 11 centers in Korea and the follow-up was tracked until January 2009.
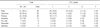
Among 156 subjects, 154 subjects were included in the evaluable-for-safety population (2 patients did not receive study treatment) and 138 subjects were included in mITT population (8 subjects had no target lesion at baseline; 8 subjects had no tumor assessment after baseline, and hence could not provide RECIST data). Patients' demographics and characteristics at baseline for the mITT population are summarized in Table 1. Breifly, patients had a median age of 64 years old (52.2% were <65 years old) and as anticipated by the entry criteria, the majority were female (87.0%), had adenocarcinoma histology (87.7%) and were non-smokers (84.8%). EGFR mutation was determined to be 109 patients. Among these patients, 59 were found to have EGFR mutation-positive, 50 were found to have EGFR mutation-negative.
The patients were administered gefitinib for approximately 5.8 months and the maximum treatment period was 18.7 months.
Of the 138 patients (mITT population), one subject had a best response of CR and 42 subjects had best responses of PR, resulting in an ORR of 31.2% (95% CI 23.6~39.6) (Table 2). In addition, disease control rate was observed 69.6% (96/138).
The degree of tumor shrinkage per individual patient as well as the number of patients who had tumor shrinkage was presented in Figure 1. This figure displayed the maximum percentage of change in the sum of the longest dimensions from baseline, and included patients who had at least one post-baseline tumor assessment. From this, changes in tumor size did not require confirmation as would necessary be by RECIST for objective response. Subgroup analysis found that ORR showed higher significant in patient groups whose tumors were EGFR mutation-positive compared with EGFR mutation-negative (45.8% vs. 14.0%, p=0.0004). There were no statistically significant differences in ORR between male and female, non-smokers and smokers, and adenocarcinoma and other histological subtypes (Table 3).
In the mITT population, progression of disease or death occurred in 100 cases with the median PFS was 5.7 months (95% CI, 3.9~8.4 months) (Figure 2). As death occurred in only 38 cases, median OS could not be derived (Figure 3). The 6-month PFS and OS were 49.6% and 87.9%, respectively. EGFR mutation-positive status showed a strong
relationship with PFS (Figure 4) and OS (Figure 5) among subjects with EGFR mutation compared with others predictors. The 12-month PFS and OS were 23.3% and 69.4%, respectively.
In overall FACT-L score, the rate of improvement with the change of points ≥6 was 43.6% (41/94 patients) (Table 4). The symptom improvement was evaluated by the LCS of FACT-L. Patients who had a change at any time of at least 2 points difference from baseline were counted as having symptom improvement. The symptom improvement rate was 51.6%.
Among 154 evaluable-for-safety patients, 141 (91.6%) reported to have AEs including 105 (68.2%) with AEs considered to be treatment-related. Serious adverse events (SAEs) were reported by 25 subjects (16.2%) and were considered treatment-related in 5 subjects (3.2%). Discontinuation due to AEs occurred in 15 subjects (9.7%) and 5 of those AEs were considered treatment-related (3.2%). SAEs leading to death occurred in 11 subjects (7.1%), 2 were considered treatment-related (1.3%; both due to interstitial lung disease). Grade 3 or 4 AEs were reported in 26 subjects (16.9%) of which 9 subjects (5.8%) were considered treatment-related (Table 5).
The most frequently reported AEs related to the treatment were rash (33.1%), diarrhea (23.4%), and pruritus (15.6%), which were mostly Common Terminology Criteria grade 1 or 2.
This Phase IV observational study was conducted targeting previously treated advanced NSCLC in Korea. Also those who had either EGFR mutation-positive or who met two of three clinical characteristics (adenocarcinoma histology, female or non-smoker) that have been typically associated with improved response to gefitinib were enrolled in this study. This study confirmed that the presence of an EGFR mutation was associated with improved response and PFS with gefitinib. Interestingly, there were no significant differences in ORR between male and female, non-smokers and smokers, and adenocarcinoma and other histological subtypes.
The IPASS demonstrated superiority in PFS for gefitinib over carboplatin and paclitaxel combined treatment in clinically selected chemonaïve patients with advanced NSCLC in Asia. Importantly, IPASS demonstrated EGFR mutation status to be a strong predictive biomarker for the efficacy of gefitinib12. The result demonstrated that patients with EGFR mutation had longer PFS with gefitinib alone than carboplatin and paclitaxel combined (hazard ratio [HR], 0.48; 95% CI, 0.36~0.64; p<0.001), while patients without EGFR mutation had longer PFS with carboplatin and paclitaxel combined than gefitinib alone (HR, 2.85; 95% CI, 2.05~3.98; p <0.001).
In patients with previous treatment of NSCLC, the randomized Phase III INTEREST trial showed non-inferiority of gefitinib in OS rate relative to docetaxel. Tumor response rate and PFS were also similar between the treatments. More patients had QoL improvements with gefitinib compared with docetaxel7. In the Phase II Iressa Dose Evaluation in Advanced Lung Cancer (IDEAL) studies, 250 mg gefitinib and 500 mg gefitinib showed similar efficacy in response rate and survival rate with the patients who had received prior chemotherapy regimens with relapsed or recurrent NSCLC. As expected, adverse effects observed to be generally mild and less in 250 mg dose group. In IDEAL 1, the significant association was detected for 3 of 22 factors: female, tumor histology, and previous immuno/biologic treatment, while female gender was the only factor associated with tumor response, giving the response as female 19%, male 3% in IDEAL 214,15. According to the Food and Drug Administration (FDA) drug approval summary, it was observed to be higher response rate for non-smokers compared with smokers in refractory or intolerant patients to platinum based and docetaxel16.
Meanwhile, in a retrospective analysis, EGFR mutation and chemonaïve were associated with improved clinical response to gefitinib. However, there was no significant difference in either OS rate or progression free survival rate-between the chemonaïve group and the chemotherapy previous treatment group17.
Based on the above results, we recruited patients with either a positive EGFR mutation or 2 or more of the following predictive factors: adenocarcinoma, female gender, and non-smoking history in present study. Patients received gefitinib 250 mg daily and were followed up to 6 months after the last patient was enrolled. The overall response rate to gefitinib treatment was 31.2% (n=43), where CR was 0.7% (n=1) and PR was 30.4% (n=42). The subgroup analysis found EGFR mutation to be predictive of improved response to gefitinib. This result was consistent with those of previously conducted studies2-4. However, in contrast to previous studies, the characteristics of gender, smoking history and/or tumor histology showed no significant association with increased responsiveness to gefitinib. The progression of disease or death occurred in 100 cases and the estimated median PFS was 5.7 months.
Toxicity results demonstrated that the most common AEs were mild or moderate and these were consistent with the known toxicity profile of gefitinib from previously conducted studies.
Compared with previous studies with gefitinib, this SELINE study reported the favorable results, which may reflect the selection criteria and hence the characteristics of the recruited patients; majority were female, non-smokers, and has adenocarcinoma histology. This study included relatively small numbers for male, smokers, and non-adenocalcinoma histology. Although these characteristics were known to associate with less response than their counterparts, there has not been driven a consensus yet. Therefore, we included them in this study.
There were some limitations in this study. First, the analyses were performed based on data that was collected by the cut-off date, and was not enable to complete the analysis of OS rate. Second, although the results of the present study show that gefitinib improved tumor response and favorable tolerability, they come from the selected patients as pointed out before. Third, it was observational study with small subjects and lack of control group. Thus, the results of current study suggest that gefitinib may consider to a second-line treatment in patients with EGFR mutations in their tumours but not be extrapolated to general population.
Gefitinib was observed in anti-tumor activity with favorable tolerability profile as a second-line therapy in these selected Korean patients. When looking at EGFR mutation status, EGFR mutation-positive showed strong association with gefitinib by greater response and prolonged PFS, compared with EGFR mutation-negative. Taken together, the results of current study were evidence to support performing the further randomized controlled studies.
Figures and Tables
Figure 1
Waterfall plot of individual patient tumor response. EGFR: epidermal growth factor receptor; SLD: sum of the longest dimensions; NA: not available.

Figure 4
Kaplan-Meier curve for progression-free survival by epidermal growth factor receptor (EGFR) mutation status. NA: not available.

Figure 5
Kaplan-Meier curve for overall survival by epidermal growth factor receptor (EGFR) mutation status. NA: not available.

Table 4
Overall FACT-L and symptom improvement rates

Values are presented as number (%).
*Overall FACT-L improvement rate: The overall FACT-L improvement rate was considered to be improvement when the change of point ≥6 in the summed FACT-L score at any 8 weeks during the study period. †Overall LCS improvement rate: The overall LCS improvement rate was considered to be improvement when the change of point ≥2 in the summed LCS score at any 8 weeks during the study period.
FACT-L: Functional Assessment of Cancer Therapy-Lung; CI: confidence interval; LCS: lung cancer subscale.
References
1. Jang TW, Oak CH, Chang HK, Suo SJ, Jung MH. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Korean J Intern Med. 2009. 24:48–54.
2. Zhang XT, Li LY, Mu XL, Cui QC, Chang XY, Song W, et al. The EGFR mutation and its correlation with response of gefitinib in previously treated Chinese patients with advanced non-small-cell lung cancer. Ann Oncol. 2005. 16:1334–1342.
3. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005. 23:2493–2501.
4. Han SW, Hwang PG, Chung DH, Kim DW, Im SA, Kim YT, et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int J Cancer. 2005. 113:109–115.
5. Hong J, Kyung SY, Lee SP, Park JW, Jung SH, Lee JI, et al. Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer. Korean J Intern Med. 2010. 25:294–300.
6. Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin. 2006. 22:561–573.
7. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005. 366:1527–1537.
8. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.
9. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–1500.
10. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004. 101:13306–13311.
11. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011. 29:2866–2874.
12. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008. 372:1809–1818.
13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.
14. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003. 21:2237–2246.
15. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003. 290:2149–2158.
16. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003. 8:303–306.
17. Wu JY, Yu CJ, Yang CH, Wu SG, Chiu YH, Gow CH, et al. First- or second-line therapy with gefitinib produces equal survival in non-small cell lung cancer. Am J Respir Crit Care Med. 2008. 178:847–853.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download