Abstract
Purpose
An imbalance of cell proliferation and cell apoptosis is an important mechanism in carcinogenesis. Capase 3, survivin and p53 have been identified as important members of the apoptotic related proteins. This study evaluated the proliferating cell nuclear antigen(PCNA), apoptosis, apoptotic related protein such as capase 3, survivin and p53 using urethane-induced mouse lung carcinogenesis, which provides reproducible steps from hyperplasia to adenocarcinoma.
Methods
Urethane was administered to the ICR mice through an intra-peritoneal injection, The mice were sacrificed at 5, 15, and 25 weeks after urethane intervention. The sequential morphological changes and immunohistochemical expression of PCNA, apoptosis, capase 3, survivin, and p53 were examined during mouse lung carcinogenesis.
Results
During carcinogenesis, the sequential histological changes were observed from hyperplasia of type II pneumocytes, to anadenoma, and ultimately to an overt adenocarcinoma. The PCNA Labeling index (LI) was 9.6% in hyperplasia, 23.2% in adenoma, and 55.7% in adenocarcinoma, respectively. The apoptotic LI was 0.24% in hyperplasia, 1.25% in adenoma, and 5.27% in adenocarcinoma. A good correlation was observed between the PCNA LI and apoptotic LI. The expression of caspase 3 was remarkable- i.e., 46.7% in adenocarcinoma, in contrast to 15% in hyperplasia and 16% in adenoma. Survivin was detected weakly in the alveolar hyperplasia and showed an increasing expressional pattern in adenoma and adenocarcinoma. p53 expression was detected only in the adenocarcinoma lesions with an expression rate of 13.3%. The level of caspase 3 expression correlated with the increase in the apoptotic index. The positive expression of caspase 3 was associated with an increased apoptotic index.
Figures and Tables
Figure 1
A. Gross finding of urethane-induced lungs, which shows multiple masses. B, C. Sequential change in urethane-induced lung lesions, showing adenoma (Figure 1B) and infiltrating adenocarcinoma (Figure 1C)(Hematoxylline & Eosin Stain, ×200). D. Immunohistochemical reactivity for PCNA in adenoma (×200). E. TUNNEL stain shows occasional nuclear staining in adenocarcinoma of lung (×200). F, G, H. Immunohistochemical reactivity for capase 3 (Figure 1F), p53 (Figure 1G) and survivin protein (Figure 1H) showing positive cytoplasmic staining (×200).
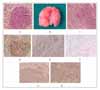
Table 2
PCNA and apoptotic labeling index of the mice lung lesions during urethane-induced carcinogenesis

References
1. Zang EA, Wynder AL. Cumulative tar exposure. A new index for estimating lung cancer risk among cigarette smokers. Cancer. 1992. 70:69–76.
2. Foley JF, Anderson MW, Stoner GD, Gaul BW, Hardisty JF, Maronpot RR. Proliferative lesions of the mouse lung: progression studies in strain A mice. Exp Lung Res. 1991. 17:157–168.
3. Malkinson AM. Pulmonary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992. 52:2670s–2676s.
4. Re FC, Manenti G, Borrello MG, Colombo MP, Fisher JH, Pierotti MA, et al. Multiple molecular alterations in mouse lung tumors. Mol Carcinog. 1992. 5:155–160.
5. Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994. 266:1821–1828.
6. Robbins BA, Vega D, Ogata K, Tan EM, Nakamura RM. Immunohistochemical detection of proliferating cell nuclear antigen in solid human malignancies. Arch Pathol Lab Med. 1987. 111:841–845.
7. Cox LS. Multiple pathways control cell growth and transformation: overlapping and independent activities of p53 and p21. J Pathol. 1997. 183:134–140.
8. Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999. 113:1076–1081.
9. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992. 119:493–501.
10. Altieri DC, Marchisio C. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999. 79:1327–1333.
11. Alnemri ES, Livinston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996. 87:171.
12. Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53 dependent apoptotic pathway. Oncogene. 2002. 21:2613–2622.
13. Chung JH, Jang JJ, Lee MJ, Ham EK. Altered retinoblastoma protein expression and proliferative activity in urethane induced mouse lung tumorigenesis. Cancer Res Treat. 2002. 34:258–263.
14. Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxillary proteins of DNA polymerase-delta. Nature. 1987. 326:515–517.
15. Morris GF, Mathews MB. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989. 264:13856–13864.
16. Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997. 14:629–640.
17. Tormanen U, Eerola AK, Rainio P, Vahakangas K, Soini Y, Sormunen R, et al. Enhanced apoptosis predicts shortened survival in non-small cell lung carcinoma. Cancer Res. 1995. 55:5595–5602.
18. Lipponen PK, Aaltomaa S. Apoptosis in bladder cancer as related to standard prognostic factors and prognosis. J Pathol. 1994. 173:333–339.
19. Shibata MA, Maroulakou IG, Jorcyk CL, Gold LG, Ward JM, Green JE. p53 independent apoptosis during mammary tumor progression in C(3)/SV 40 large T antigen transgenic mice; Supression of apoptosis during the transition from preneoplasia to carcinoma. Cancer Res. 1996. 56:2998–3003.
20. Sela B. Survivin: anti-apoptosis proteins and a prognostic markers for progression and recurrence. Harefuah. 2002. 141:103–107.
21. Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, et al. Survivin promotors cell proliferation in human hepatocellular carcinoma. Hepatology. 2000. 31:1080–1085.
22. Lin LJ, Zheng CQ, Jin Y, Ma Y, Jiang WG, Ma T. Expression of survivin protein in human colorectal carcinogenesis. World J Gastroenterol. 2003. 9:974–977.
23. Zhang X, Dong N, Yin L, Cai N, Ma H, You J, et al. Hepatitis B virus X protein upregulates survivin expression in hepatoma tissues. J Med Virol. 2005. 77:374–381.
24. Akyurek N, Memis L, Ekinci O, Kokturk N, Ozturk C. Survivin expression in pre-invasive lesions and non-small cell lung carcinoma. Virchows Arch. 2006. 449:164–170.
25. Hsia JY, Chen CY, Chen JT, Hsu CP, Shai SE, Yang SS, et al. Prognostic significance of caspase-3 expression in primary resected esophageal squamous cell carcinoma. Eur J Surg Oncol. 2003. 29:44–48.
26. Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004. 10:1984–1988.
27. Isobe N, Onodera H, Mori A, Shimada Y, Yang W, Yasuda S, et al. Caspase-3 expression in human gastric carcinoma and its clinical significance. Oncology. 2004. 66:201–209.
28. Hall PA, Lane DP. p53 in tumor pathology: can we trust immunohistochemistry? J Pathol. 1994. 172:1–4.
29. Cazorla M, Hernandez L, Fernandez PL, Fabra A, Peinado MA, Dasenbrock C, et al. Ki-ras gene mutations and absence of p53 gene mutations in spontaneous and urethane-induced early lung lesions in CBA/J mice. Mol Carcinog. 1998. 21:251–260.
30. Horio Y, Chen A, Rice P, Roth JA, Malkinson AM, Schrump DS. Ki-ras and p53 mutations are early and late events, respectively, in urethane-induced pulmonary carcinogenesis in A/J mice. Mol Carcinog. 1996. 17:217–223.
31. Ohno J, Horio Y, Sekido Y, Hasegawa Y, Takahashi M, Nishizawa J, et al. Telomerase activation and p53 mutations in urethane-induced A/J mouse lung tumor development. Carcinogenesis. 2001. 22:751–756.
32. Mori I, Yasuhara K, Hayashi SM, Nonoyama T, Nomura T, Yanai T, et al. Aberrant expression of cyclin D1 in pulmonary proliferative lesions induced by high doses of urethane in transgenic mice carrying the human prototype c-H-ras gene. J Vet Med Sci. 2001. 63:261–268.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download