This article has been corrected. See "Erratum to: Elevated Serum Uric Acid in Benign Convulsions with Mild Gastroenteritis in Children" in Volume 16 on page 181.
Abstract
Background and Purpose
To identify whether serum uric acid levels are significantly higher in patients with benign convulsion associated with mild gastroenteritis (CwG) than in patients with acute gastroenteritis.
Methods
This retrospective study compared the serum levels of uric acid between CwG, acute gastroenteritis, and febrile seizure after correcting for the varying degree of mild dehydration using serum HCO3− levels. We also compared the serum uric acid levels between patients with CwG and febrile seizures in order to exclude the effect of seizures on uric acid.
Results
This study included 154 CwG patients (age range 0.73–3.19 years), 2,938 patients with acute gastroenteritis, and 154 patients with febrile seizure. The serum uric acid level was significantly higher in CwG patients than in patients with acute gastroenteritis [9.79±2.16 mg/dL vs. 6.04±2.3 mg/dL (mean±SD), p<0.001]. This difference was also significant after correcting for dehydration. The serum uric acid level was significantly higher in CwG patients than in dehydration-corrected acute gastroenteritis patients (9.79±2.16 mg/dL vs. 6.67±2.48 mg/dL, p<0.001). The serum uric acid level was not elevated in patients with febrile seizure.
Benign convulsions associated with mild gastroenteritis (CwG) was first reported by Morooka in 19821 and has subsequently been reported mainly in East Asia, with only a few recent reports from Europe and USA.234 CwG is defined as afebrile seizure accompanied by acute gastroenteritis symptoms such as diarrhea in healthy patients, without evidence of central nervous system infection, electrolyte abnormalities, or moderate-to-severe dehydration to induce seizures.5 The prognosis of CwG is good, and it can be treated as a situation-related seizure.678 This means that the early diagnosis of CwG does not require expensive tests such as brain magnetic resonance imaging or electroencephalography, and long-term anticonvulsant therapy can be avoided when initially diagnosing CwG.
However, unlike febrile seizure, which is another situation-related seizure in children, CwG is not accompanied by fever, and some patients will not have symptoms of gastroenteritis such as diarrhea at the onset of seizures.910 The diagnosis of CwG is instead heavily reliant on the history of the patient and the specific characteristics of gastroenteritis and the seizures. Moreover, there is no specific biomarker for CwG that will aid its diagnosis when the physician is uncertain. Therefore, diagnosing CwG can be difficult in the early phases of the disease.
Since serum uric acid level was added to the routine blood chemistry battery of our hospital we frequently observed highly elevated serum uric acid levels in CwG patients, whereas the serum uric acid level of gastroenteritis patients without seizures have been normal or only mildly elevated. Moreover, there have been a few reports of the serum uric acid being higher in CwG patients than in the patients with gastroenteritis only.1112
The purpose of the present study was to determine whether serum uric acid levels are significantly higher in patients with CwG than in patients with gastroenteritis only. We also aimed to reveal whether any such elevation was due to either dehydration or seizures.
This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB No. B-1710-424-102).
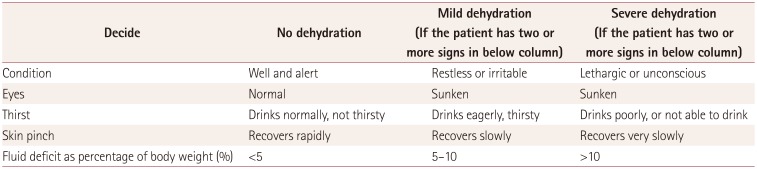
The study population consisted of patients diagnosed with acute gastroenteritis, febrile seizure, or CwG from March 2007 to February 2016 at Seoul National University Bundang Hospital (SNUBH). These patients were identified using the clinical data warehouse (CDW) system at SNUBH. CwG was defined as one or more afebrile seizures with acute gastroenteritis during the disease period without any evidence of central nervous system infection, dehydration, or electrolyte imbalance.5 Since these criteria lack detailed and strict information on dehydration, we excluded patients with moderate-to-severe dehydration based on the WHO guideline13 (Table 1) and laboratory findings, in accordance with previous studies.141516 None of the patients suspected of CwG showed moderate-to-severe dehydration. Patients with epilepsy, congenital central nervous system disorders, or electrolyte imbalance that could provoke seizures were excluded.
Clinical information was collected regarding the age at onset, sex, timing and frequency of diarrhea and seizures, and past medical history such as epilepsy and congenital central nervous system disease. Serum and laboratory data including uric acid, sodium, potassium, chloride, calcium, magnesium, glucose, bicarbonate (HCO3−), blood urea nitrogen, and creatinine were reviewed to exclude patients with electrolyte imbalance or hypoglycemia that could cause seizures. Laboratory examinations were performed at the time of the visiting the emergency room (usually after the seizure) in most cases. The main purpose of this study was to identify any differences in serum uric acid levels between CwG patients and patients with acute gastroenteritis without seizures. We also aimed to reveal whether the findings resulted from dehydration or a postictal phenomenon.1718 To achieve this goal, we compared serum uric acid levels between CwG patients and all acute gastroenteritis patients without seizures. We further analyzed the difference in serum uric acid levels after correcting for dehydration in order to exclude the confounding effect of dehydration on the serum uric acid level. This correction was performed using HCO3−, which is a wellknown indicator of dehydration severity.1920 We randomly selected four patients with simple acute gastroenteritis who matched the HCO3− level, age, and sex of a single CwG patient. Lastly, we compared the serum uric acid levels of the CwG patients with those in a sex- and age-matched (1:1) control group with febrile seizure to determine whether any difference was the result of seizures. We used the Kolmogorov-Smirnov and Shapiro-Wilk tests to check if the continuous variables conformed to a normal distribution. Continuous variables were compared using the independent-sample t-test, while categorical variables were compared using the chi-squared test. The results were analyzed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA), and a p value less than 0.05 was considered statistically significant. Receiver operating characteristics (ROC) analysis was performed using the pROC package of R software (version 3.4.0, R Foundation, Vienna, Austria) to calculate the area under the ROC curve and the serum uric acid cutoff value that optimally distinguished CwG from acute gastroenteritis patients. ROC curves were constructed over the range of all observed values, and corresponding confidence intervals were computed. The optimal threshold value corresponds to the best sum of sensitivity and specificity (Youden index).21
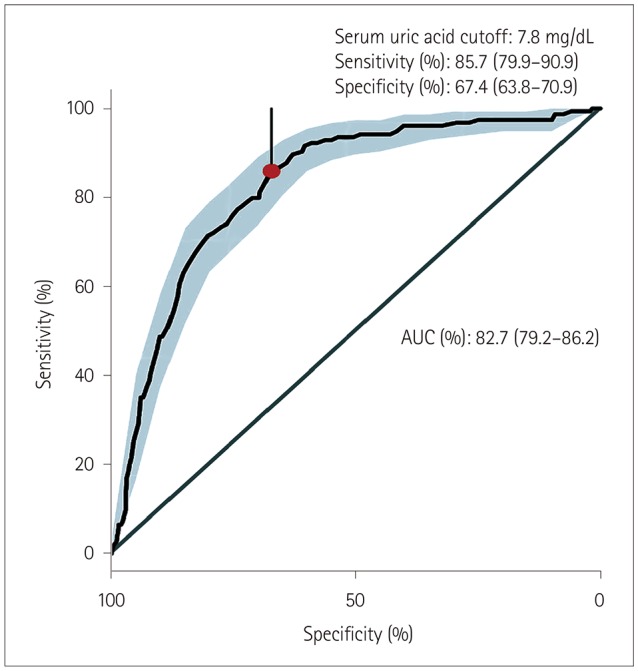
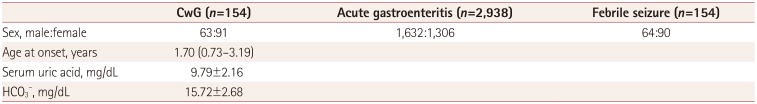
We identified 154 CwG patients (male:female=63:91, age range 0.73–3.19 years) through a CDW search of diagnoses, and performed detailed reviews of their electronic medical records. We identified 2,938 patients (1,632 male and 1,306 female) with acute gastroenteritis during the same time period whose age distribution was the same as that of the CwG patients. The same number of patients with febrile seizure was identified by matching the sex and age of the CwG patients. All CwG patients experienced a single seizure event before a blood test was performed, and none of them experienced prolonged seizures lasting >15 minutes. All patients with febrile seizure showed simple febrile seizure. Laboratory testing was performed within 12 hours after the first seizure for both the CwG and febrile seizure groups. The age, sex ratio, serum uric acid, and HCO3− levels of the patients are listed in Table 2. The serum uric acid level of the CwG patients was 9.79±2.16 mg/dL [mean±SD; 95% confidence interval (CI)=9.44–10.13 mg/dL] and that of the acute gastroenteritis patients without seizure was 6.04±2.36 mg/dL (95% CI=5.96–6.13 mg/dL). The uric acid level in each group was higher than the normal range (reference range at SNUBH=1.80–5.00 mg/dL) and the uric acid level of the CwG patients was significantly higher than that of the patients with acute gastroenteritis without seizures) (p<0.001) (Fig. 1). As mentioned above, we randomly selected four patients with acute gastroenteritis and the same HCO3− value, sex, and age as those of a CwG patient. We compared the HCO3−-matched, 4:1 paired group with an acute gastroenteritis patient with CwG patients. The serum uric acid level of the CwG patients (9.79±2.16 mg/dL) was significantly higher (p<0.001) than that of 616 HCO3−-corrected acute gastroenteritis patients (6.67±2.48 mg/dL, 95% CI=6.47–6.87 mg/dL). The serum uric acid level was higher in CwG patients even after randomly correcting for the effect of dehydration using the serum HCO3− level. The distributions of the serum uric acid levels in the HCO3−-corrected acute gastroenteritis group and CwG group are shown in Fig. 2. The serum uric acid level of patients with febrile seizure was 4.30±1.02 mg/dL (95% CI=4.14–4.46 mg/dL), which falls within the normal range (Fig. 1). We also found that the uric acid did not increase after the seizure in patients with febrile seizure, which suggests that the elevation of serum uric acid in CwG is not a postictal phenomenon. ROC analysis revealed that the optimal serum uric acid cutoff value for distinguishing between CwG and HCO3−-matched acute gastroenteritis was 7.8 mg/dL, which gave a sensitivity of 85.7% (95% CI=79.9–90.9%), a specificity of 67.4% (95% CI=63.8–70.9%), and an area under the curve of 82.7% (95% CI=79.2–86.2%), as shown in Fig. 3. This serum uric acid level may be useful for diagnosing CwG in its early stages.
The key findings in this study were as follows: 1) the serum uric acid level was significantly higher in patients with CwG than in those with acute gastroenteritis, 2) this elevation was also present after correcting for dehydration, which supports that this significant result was not biased by the severity of dehydration, and 3) this finding was not observed in the postictal state of febrile seizure patients, which also supports that this elevation was not the result of seizure. Our findings confirm the hypothesis that we raised based on our observation from everyday practice-that the serum uric acid level of CwG patients is higher than that of acute gastroenteritis patients without seizure. Although only a few studies have addressed this issue, our findings are concordant with previous studies finding higher uric acid levels in CwG patients than in patients with acute gastroenteritis alone. One study from Japan compared 20 patients with CwG and 30 patients with acute gastroenteritis, who had similar degrees of metabolic acidosis, and found that the serum uric acid level was significantly higher in the CwG group (10.0±2.2 mg/dL) than in the patients with acute gastroenteritis (8.8±5.2 mg/dL).12 Another study found that the serum uric acid level was higher in 27 CwG patients (8.75±2.31 mg/dL) than in 188 patients with simple gastroenteritis (5.91±2.45 mg/dL).11 We similarly found that the serum uric acid level was significantly higher in patients with CwG than in those without seizure from a larger cohort of 154 CwG patients.
The second question that we aimed to address was whether this higher uric acid level was due to dehydration as a result of diarrhea, vomiting, and reduced oral intake. It has been reported that the serum uric acid level increases in the presence of severe dehydration,22 and so we attempted to correct for any difference in the degree of dehydration between the CwG and acute gastroenteritis groups. Accurately determining the degree of dehydration requires the application of laboratory data together with the Clinical Dehydration Scale (CDS) using the symptoms and signs of patients.1920 Since patients can only be classified semiquantitatively into mild, moderate, or severe by applying the CDS, we needed a continuous scale that could precisely reflect the degree of dehydration when performing this correction. We therefore quantified the degree of dehydration using the serum HCO3−, which is known to accurately reflect the severity of dehydration as a single laboratory result.192023 To correct for the effects of a difference in dehydration on the uric acid level in the CwG group, we created a dehydration-corrected control group without seizure by randomly selecting an additional 4:1 HCO3−-matched control group comprising patients with acute gastroenteritis. We confirmed that serum uric acid level of the CwG patients was significantly higher than that of the acute gastroenteritis patients without seizures even after correcting for the degree of dehydration. This is consistent with the uric acid level being higher in patients with CwG than in acute gastroenteritis patients with a similar severity of dehydration. This finding also suggests that the significantly higher level of serum uric acid level was not a biased result resulting from a different degree of dehydration in the CwG patients.
There have been reports of the serum uric acid level increasing after prolonged seizures in humans.1724 We therefore investigated whether the higher level of serum uric acid in CwG patients was the result of seizures by comparing the uric acid levels of patients after febrile seizures. Almost all (n=149) of the 152 patients with CwG and all of the patients with febrile seizure visited the emergency room immediately after their seizure, which enabled us to assess the effect of the seizure on the serum uric acid level. Serum uric acid was not elevated in the patients after a febrile seizure, which confirms that the abnormal elevation of the serum uric acid level in the CwG patients was not a phenomenon that occurred after a seizure. Overall, our findings confirm that significantly higher serum uric acid levels are observed in CwG patients compared to acute gastroenteritis patients without seizure and their levels are not confounded by the degree of dehydration or a postictal phenomenon.
As mentioned above, we frequently observe markedly elevated serum uric acid levels in patients with CwG in our everyday practice. We can use this finding as an additive laboratory marker for CwG, especially in patients with multiple seizures or those without the overt clinical manifestation of acute gastroenteritis. Based on our findings, a serum uric acid level above 7.8 mg/dL can serve as a helpful marker suggestive of CwG over other epileptic diseases. Additionally, it could help physicians to anticipate seizures and prepare for possible events when encountering a patient with acute gastroenteritis with a markedly elevated serum uric acid level.
Our findings can also provide a helpful suggestion for further basic research into the mechanism underlying seizure generation. Uric acid has been considered metabolic waste in the past, but recent studies have shown that it affects not only gout but also ischemic heart disease and neurological diseases.25262728 In particular, the metabolites of uric acid can enhance the activity of the nervous system and act as an inflammatory substance.29 Although various functions of uric acid are known, only a few studies have investigated the association between uric acid and seizures. An increase in serum uric acid levels in mice is reportedly associated with an increase in generalized seizures.18 In the present study, the serum uric acid levels were significantly higher in CwG patients. These clinical observations suggest that further basic research is needed into the relationship between uric acid and seizures.
While geographical variations in CwG have not been reported, it is mainly found in East Asian countries such as Japan, Taiwan, and Korea, and its prevalence is thought to vary with race.3 In addition, the occurrence of seizures and the increased uric acid levels differ between individuals. These observations suggest the presence of a genetic influence on the occurrence of CwG. It remains difficult to attribute the pathophysiology of CwG to a single gene. However, well known to be genes associated with elevations of the serum uric acid level.303132 Dysfunction of ABCG2 in gastroenteritis patients with elevation of serum uric acid was detected recently, and so this is one of the causes of serum uric acid elevation in gastroenteritis.33 Clarifying the elevation of uric acid levels in CwG patients will require further studies of the genes related to uric acid metabolism.
This study enrolled a large number of patients with CwG and acute gastroenteritis. Also, because it was performed at a single institutional, patients and controls were selected based on consistent criteria and comparisons of the obtained laboratory values are likely to be relatively reliable. However, some clinical records could not be identified because of the limitation of this study having a retrospective design. Therefore, there were limitations in analyzing other factors that could affect the occurrence of seizures. Also, insufficient clinical information in the CDS reduced our ability to determine the degree of dehydration of individual patients, and the degree of dehydration could not be assessed more accurately by using both CDS and laboratory data.
We have found that the serum uric acid level is significantly elevated in patients with CwG. This finding strongly suggests that if seizure occurs in children aged 6–36 months with gastroenteritis, a significantly increased serum uric acid levels can serve as a helpful indicator for diagnosing CwG in its early phase.
Notes
Author contributions:
Conceptualization: Ki Joong Kim, Byung Chan Lim, Hunmin Kim.
Data curation: Woojoong Kim, Il Han Yoo, Jaeso Cho.
Formal analysis: Il Han Yoo, Hunmin Kim.
Investigation: Hee Hwang.
Methodology: Hunmin Kim, Il Han Yoo.
Project administration: Jong-Hee Chae.
Resources: Hunmin Kim.
Supervision: Ki Joong Kim, Jieun Choi.
Validation: Hunmin Kim.
Visualization: Jaeso Cho, Il Han Yoo, Hunmin Kim.
Writing—original draft: Il Han Yoo, Hunmin Kim, Woojoong Kim.
Writing—review & editing: Il Han Yoo, Hunmin Kim, Hee Hwang.
References
1. Morooka K. Convulsions and mild diarrhea. Shonika. 1982; 23:134–137.
2. DiFazio MP, Braun L, Freedman S, Hickey P. Rotavirus-induced seizures in childhood. J Child Neurol. 2007; 22:1367–1370. PMID: 18174553.

3. Narchi H. Benign afebrile cluster convulsions with gastroenteritis: an observational study. BMC Pediatr. 2004; 4:2. PMID: 15005806.

4. Verrotti A, Nanni G, Agostinelli S, Parisi P, Capovilla G, Beccaria F, et al. Benign convulsions associated with mild gastroenteritis: a multicenter clinical study. Epilepsy Res. 2011; 93:107–114. PMID: 21146369.

5. Castellazzi L, Principi N, Agostoni C, Esposito S. Benign convulsions in children with mild gastroenteritis. Eur J Paediatr Neurol. 2016; 20:690–695. PMID: 27292317.

6. Specchio N, Vigevano F. The spectrum of benign infantile seizures. Epilepsy Res. 2006; 70 Suppl 1:S156–S167. PMID: 16837167.

7. Tanabe T, Hara K, Kashiwagi M, Tamai H. Classification of benign infantile afebrile seizures. Epilepsy Res. 2006; 70 Suppl 1:S185–S189. PMID: 16814520.

8. Verrotti A, Moavero R, Vigevano F, Cantonetti L, Guerra A, Spezia E, et al. Long-term follow-up in children with benign convulsions associated with gastroenteritis. Eur J Paediatr Neurol. 2014; 18:572–577. PMID: 24780603.

9. Durá-Travé T, Yoldi-Petri ME, Molins-Castiella T, Souto-Hernández S, Aguilera-Albesa S. Infantile convulsions with mild gastroenteritis: epidemiological and clinical characteristics and outcome. Rev Neurol. 2010; 51:12–18. PMID: 20568063.
10. Komori H, Wada M, Eto M, Oki H, Aida K, Fujimoto T. Benign convulsions with mild gastroenteritis: a report of 10 recent cases detailing clinical varieties. Brain Dev. 1995; 17:334–337. PMID: 8579220.

11. Chae SH, Rhee M, Kim YC, Kim SS. The relationship between serum uric acid level and benign convulsions with mild gastroenteritis. J Korean Child Neurol Soc. 2014; 22:191–194.
12. Tsujita Y, Matsumoto H, Nakamura Y, Nonoyama S. Analysis of the blood and serum biochemistry findings in patients demonstrating convulsion with mild gastroenteritis. No To Hattatsu. 2011; 43:282–284. PMID: 21800691.
13. World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva: World Health Organization;2005.
14. Kang B, Kim DH, Hong YJ, Son BK, Kim DW, Kwon YS. Comparison between febrile and afebrile seizures associated with mild rotavirus gastroenteritis. Seizure. 2013; 22:560–564. PMID: 23642407.

15. Kawano G, Oshige K, Syutou S, Koteda Y, Yokoyama T, Kim BG, et al. Benign infantile convulsions associated with mild gastroenteritis: a retrospective study of 39 cases including virological tests and efficacy of anticonvulsants. Brain Dev. 2007; 29:617–622. PMID: 17544607.

16. Li T, Hong S, Peng X, Cheng M, Jiang L. Benign infantile convulsions associated with mild gastroenteritis: an electroclinical study of 34 patients. Seizure. 2014; 23:16–19. PMID: 24125788.

17. Makki N, Hajj G, Schmidt GA. Seizure-induced acute urate nephropathy: case report and review. Chest. 2013; 144:666–669. PMID: 23918111.
18. Thyrion L, Raedt R, Portelli J, Van Loo P, Wadman WJ, Glorieux G, et al. Uric acid is released in the brain during seizure activity and increases severity of seizures in a mouse model for acute limbic seizures. Exp Neurol. 2016; 277:244–251. PMID: 26774005.

19. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004; 291:2746–2754. PMID: 15187057.

20. Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997; 13:179–182. PMID: 9220501.

21. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. PMID: 21414208.

22. Kuge R, Morikawa Y, Hasegawa Y. Uric acid and dehydration in children with gastroenteritis. Pediatr Int. 2017; 59:1151–1156. PMID: 28714223.

23. Tam RK, Wong H, Plint A, Lepage N, Filler G. Comparison of clinical and biochemical markers of dehydration with the clinical dehydration scale in children: a case comparison trial. BMC Pediatr. 2014; 14:149. PMID: 24935348.

24. Warren DJ, Leitch AG, Leggett RJ. Hyperuricaemic acute renal failure after epileptic seizures. Lancet. 1975; 2:385–387. PMID: 51191.
25. Spitsin S, Markowitz CE, Zimmerman V, Koprowski H, Hooper DC. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. J Hum Hypertens. 2010; 24:359–362. PMID: 19865105.

26. Andreadou E, Nikolaou C, Gournaras F, Rentzos M, Boufidou F, Tsoutsou A, et al. Serum uric acid levels in patients with Parkinson's disease: their relationship to treatment and disease duration. Clin Neurol Neurosurg. 2009; 111:724–728. PMID: 19632030.

27. Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007; 69:1696–1700. PMID: 17954784.

28. Hamed SA, Hamed EA, Hamdy R, Nabeshima T. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res. 2007; 74:183–192. PMID: 17448640.

29. Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Tolllike receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010; 16:413–419. PMID: 20348922.

30. Matsuo H, Ichida K, Takada T, Nakayama A, Nakashima H, Nakamura T, et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci Rep. 2013; 3:2014. PMID: 23774753.

31. Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008; 83:744–751. PMID: 19026395.

32. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002; 417:447–452. PMID: 12024214.
33. Matsuo H, Tsunoda T, Ooyama K, Sakiyama M, Sogo T, Takada T, et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2. Sci Rep. 2016; 6:31003. PMID: 27571712.

Fig. 1
Boxplot of serum uric acid level of the three groups (CwG, acute gastroenteritis, and febrile seizure). *p<0.05. CwG: benign convulsions associated with mild gastroenteritis.
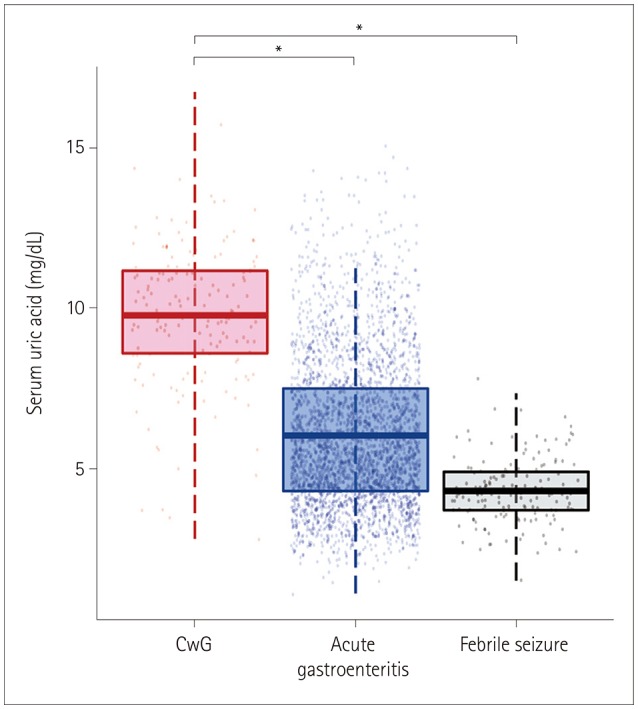
Fig. 2
Serum UA level in patients with CwG and acute gastroenteritis after 1:4 matching by HCO3− levels. A: Histogram of serum UA in CwG and HCO3− matched AGE patients. B: Distribution of serum UA in CwG and HCO−3-matched acute gastroenteritis patients shown in a density plot. Dashed lines indicate mean serum UA levels of each group (*p<0.05). CwG: benign convulsions associated with mild gastroenteritis, UA: uric acid.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download