Dear Editor,
Out of various neurological manifestations associated with non-ketotic hyperglycemia (NKH),1 speech arrest as a manifestation of seizure is very rare although visual and generalized seizures have been often reported.23 We report herein a patient with speech arrest as a manifestation of NKH-induced seizure in which technetium-99m (Tc-99m) hexamethylpropylene amine oxime (HMPAO) brain single-photon emission computed tomography (SPECT) revealed a localized seizure focus.
A right-handed, 74-year-old man who had a past medical history of type 2 diabetes mellitus (DM) and no epileptic seizure presented with several episodes of speech arrest and right lower facial spasm without motor weakness of extremities for 5 days before visit. The average duration in each episode was 2 min, during which his consciousness and auditory comprehension were considered in the normal range of characteristics. However, he could not speak out loud at all. He did not have no history of fever or recent flu-like symptoms or head injury. The patient had been prescribed valproate for 4 days after being diagnosed with focal aware seizure at a local clinic without relevant electroencephalography (EEG) findings at that time.
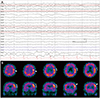
The laboratory data showed elevated serum glucose level of 446 mg/dL and hemoglobin A1c of 11.4%. The EEG showed mainly muscle artifacts due to a facial spasm, which obscured the presence of epileptiform discharges, but intermittent left frontotemporal fast rhythmic activities during ictal phase (Fig. 1A). The magnetic resonance imaging and angiography of the brain was normal. The Tc-99m HMPAO brain SPECT demonstrated hyper-perfusion at the left precentral gyrus and, middle and inferior frontal gyrus (Fig. 1B). Our patient was diagnosed with NKH-induced seizure and aggressive glucose control and oral carbamazepine was done. The patient had become seizure free since the second day after hospitalization.
We report a rare case of NKH-induced focal aware seizure presenting with right facial twitching and speech arrest. Although the pathogenesis of epileptic seizure in patients with NKH is not fully understood, it is probable that elevated serum glucose level is a proconvulsant condition.4 Hyperglyceima also increases gamma-aminobutyric acid metabolism, and thereby lowers the seizure threshold.1 Therefore, the early diagnosis and immediate blood sugar control seems to be important in case of seizure associated with NKH. However, the clinical manifestations may be obscure as in our case or other previously reported cases15 which poses a challenge to diagnosis.
Penfield and Rasmussen6 previously showed that electrical stimulation of the dominant frontal operculum, and both precentral gyri caused speech arrest. In addition, one recent functional mapping study using direct cortical stimulation showed that speech arrest is most likely to occur with stimulation of the posterior inferior frontal gyrus and precentral gyrus.7 In other words, the above mentioned areas are considered as negative motor area (NMA) of speech.
In our patient, the ictal SPECT was revealed increased perfusion in the areas consistent with the previous reports. Speech arrest was observed simultaneously with right lower facial twitching due to excitation of the motor cortex in accordance with the motor homunculus. Similarly, previous cases reported sites producing both inhibition of ongoing movements and also excitation of facial musculature.8
Although some cases of NKH-associated occipital lobe seizures diagnosed through brain SPECT had been previously reported,5 to the best our knowledge, we report the first case of NKH-induced focal aware seizure presenting with speech arrest in which the relevant anatomical regions were demonstrated by the brain SPECT.
Our report demonstrated that ictal SPECT should be considered as one of diagnostic tool in patients with a suspected NKH-induced speech arrest as an early recognition of seizure and rapid correction of blood sugar are critical.
Figures and Tables
Fig. 1
Ictal EEG and brain SPECT. A: Ictal EEG shows focal rhythmic epileptic discharges over the left frontotemporal area. B: Ictal brain SPECT shows focal hyperperfusion at left precentral gyrus (arrows) and middle and inferior frontal gyri (arrowheads). EEG: electroencephalography, SPECT: single-photon emission computed tomography.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07048873).
References
1. Hennis A, Corbin D, Fraser H. Focal seizures and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry. 1992; 55:195–197.



2. Carril JM, Guijarro C, Portocarrero JS, Solache I, Jiménez A, Valera de Seijas E. Speech arrest as manifestation of seizures in non-ketotic hyperglycaemia. Lancet. 1992; 340:1227.

3. Venna N, Sabin TD. Tonic fecal seizures in nonketotic hyperglycemia of diabetes mellitus. Arch Neurol. 1981; 38:512–514.

4. Schwechter EM, Velísková J, Velísek L. Correlation between extracellular glucose and seizure susceptibility in adult rats. Ann Neurol. 2003; 53:91–101.


5. Liu CJ, Tsai HH, Ko KY, Lu CC, Yen RF. Ictal phase perfusion SPECT of nonketotic hyperglycemia-induced parieto-occipital seizure. Clin Nucl Med. 2017; 42:e67–e68.

6. Penfield W, Rasmussen T. Vocalization and arrest of speech. Arch Neurol Psychiatry. 1949; 61:21–27.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download