Dear Editor,
The anti-myelin oligodendrocyte glycoprotein antibody (MOG-Ab) has recently been recognized as a marker for a group of CNS demyelinating diseases that are distinct from multiple sclerosis and neuromyelitis optica spectrum disorder (NMOSD) with anti-aquaporin-4 (AQP4) immunoglobulin G (IgG).1 There have been a few case reports on MOG-Ab-associated disease following viral infections such as influenza2 and Epstein-Barr virus,3 but the pathophysiological mechanism of postinfectious autoimmunity remains unclear. Here we report the first case of MOG-Ab-associated disease that was possibly triggered by a rubella infection.
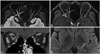
A 19-year-old man sought treatment after having experienced fever, headache, and posterior auricular/neck pain for 1 month. He presented with decreased right visual acuity and mild periocular pain that had persisted for more than 10 days. An ophthalmological examination revealed that his best-corrected visual acuity was 0.7/0.9 (right/left, in decimals and as measured using a Snellen chart) and that he had a grade 1 relative afferent pupillary defect and optic disc swelling in his right eye. Orbit MRI demonstrated T2 high signal intensities and swelling with prominent perineural enhancement along the right optic nerve. Brain MRI showed a focal T2 high signal intensity in the left thalamus (Fig. 1). Spine MRI showed no significant abnormalities.
A CSF analysis showed a red blood cell count of 0/µL, white blood cell count of 23/µL (5% neutrophils, 84% lymphocytes, and 11% monocytes), protein level of 36.7 mg/dL, and glucose level of 77 mg/dL. The patient's CSF oligoclonal band and cytology were both negative, and his IgG index was 0.72. The serum beta-2 microglobulin and lactic acid dehydrogenase levels were normal. A vasculitis workup that included analyses of antinuclear antibodies, angiotensin-converting enzymes, antineutrophil cytoplasmic antibodies, and anti-Ro/La antibodies produced no significant results.
The CSF PCRs for cytomegalovirus, JC virus, herpes simplex virus type 1/2, varicella zoster virus, and Epstein-Barr virus produced negative findings. The patient's serum exhibited positivity for IgM and IgG antibodies to the rubella virus, as measured using a chemiluminescence microparticle immunoassay (IgM titer: 2.64 IU/mL, IgG titer: 11.6 IU/mL). The results of a serum AQP4-IgG test using an indirect immunofluorescence assay were negative, while those of a serum IgG1 MOG-Ab test using a cell-based assay utilizing full-length human MOG (Radcliffe Hospital, Oxford, UK) were positive.4
The above-described findings led to suspicion of MOG-Ab-positive optic neuritis, and so intravenous methylprednisolone (1,000 mg pulse therapy for 5 days) was initiated. A followup examination performed 1 month later showed that the patient's right visual acuity had improved significantly after administering oral prednisolone at 60 mg daily, and so the steroid therapy was tapered out.
This is the first case report of a patient who developed MOG-Ab-positive optic neuritis following a presumed rubella infection. Although the presence of a rash or lymphadenopathy was uncertain at presentation, and PCR for rubella virus was not performed, the patient's 1-month history of fever and posterior auricular/neck pain prior to seeking treatment as well as his seropositivity for IgM and IgG antibodies to the rubella virus supported the presence of a recent infection. A rubella infection can occasionally manifest without a rash, and even if present, it seldom persists for more than several days and is not followed by staining or desquamation.56 In addition, our patient did not have a recent history of the measles, mumps, and rubella (MMR) vaccination, and his titer of the IgM antibody to the rubella virus was relatively high (165% of the limit for positivity). The time interval from rubella infection to optic neuritis seemed long in this case; however, there is a previous case report of optic neuritis developing more than 1 month following acute rubella infection.7
We propose that the rubella virus is able to trigger MOG-Ab-associated disease because 1) the rubella E1 protein binds to MOG on oligodendrocytes in the CNS,8 and 2) there is a high level of molecular mimicry between the rubella E2 protein and MOG.9 The E1 and E2 proteins are anchored to the external layer of the rubella virus envelope,5 and so antibodies against the rubella virus might cause demyelination of the CNS and trigger MOG-Ab-associated disease. A previous case report described a 17-year-old man with a relapsing course of acute disseminated encephalomyelitis (ADEM) following a rubella infection without a rash, although MOG-Ab was not tested in that case.10 MOG-Ab-associated disease typically presents with optic neuritis and shows a monophasic course. Nevertheless, its phenotype has recently been reported to be much broader, including an ADEM-like presentation and a relapsing disease course.11
In the prevaccination era, the incidence of rubella infection in the United States peaked in the age group of 5–9 years; however, this peak has changed to the age group of 15–24 years following the introduction of the MMR vaccination.56 It is particularly interesting that MOG-Ab is predominantly detected in pediatric or young adult patients with ADEM, recurrent optic neuritis, and AQP4-IgG-negative NMOSD.12 The overlapping age distribution between rubella cases and MOG-Ab-associated disease may suggest a shared pathophysiological process. Further experimental studies are warranted to elucidate the exact pathophysiological mechanism of MOG-Ab-associated disease.
Figures and Tables
Fig. 1
Orbit and brain MRI. Orbit MRI showed high signal intensities along the right optic nerve in a T2-weighted FLAIR image (A), and perineural enhancement in contrast-enhanced T1-weighted images (B and C). Brain MRI showed a focal high signal intensity in the left thalamus in a T2-weighted FLAIR image (D). Solid and dotted arrows indicate the areas with T2 high signal intensities and contrast enhancement, respectively.
References
1. Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015; 2:e163.

2. Amano H, Miyamoto N, Shimura H, Sato DK, Fujihara K, Ueno S, et al. Influenza-associated MOG antibody-positive longitudinally extensive transverse myelitis: a case report. BMC Neurol. 2014; 14:224.

3. Nakamura Y, Nakajima H, Tani H, Hosokawa T, Ishida S, Kimura F, et al. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol. 2017; 17:76.

4. Waters P, Woodhall M, O'Connor KC, Reindl M, Lang B, Sato DK, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015; 2:e89.

6. Evans AS. Viral infections of humans: epidemiology and control. 2nd ed. New York: Springer;1984.
7. Cansu A, Ayşe S, Şengül Ö, Kivilcim G, Tuğba HL. Bilateral isolated acute optic neuritis in a child after acute rubella infection. Jpn J Ophthalmol. 2005; 49:431–433.

8. Cong H, Jiang Y, Tien P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J Virol. 2011; 85:11038–11047.

9. Besson Duvanel C, Honegger P, Matthieu JM. Antibodies directed against rubella virus induce demyelination in aggregating rat brain cell cultures. J Neurosci Res. 2001; 65:446–454.

10. Shinoda K, Asahara H, Uehara T, Miyoshi K, Suzuki SO, Iwaki T, et al. Multiphasic acute disseminated encephalomyelitis associated with atypical rubella virus infection. Mult Scler. 2015; 21:252–254.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download