Abstract
Background and Purpose
Although traditionally regarded as spared, a range of oculomotor dysfunction has been recognized in amyotrophic lateral sclerosis (ALS) patients. ALS is nowadays considered as a neurodegenerative disorder of a third compartment comprising widespread areas of extra-motor brain including cerebellum. Our objective was to perform an observational study to examine for ocular motor dysfunction in patients with ALS and for any differences between bulbar-onset and spinal-onset patients.
Methods
Thirty two ALS patients (bulbar onset: 10, spinal onset: 22) underwent the standardized systemic evaluations using video-oculography.
Results
Oculomotor dysfunctions such as square wave jerks, saccadic dysmetria, abnormal cogwheeling smooth pursuits and head shaking and positional nystagmus of central origin have been observed in the ALS patients at a relatively early stage. Abnormal smooth pursuits and saccadic dysmetria were increased in the bulbar-onset compared to the spinal-onset (p<0.05).
Amyotrophic lateral sclerosis (ALS) is classically defined by the degeneration of upper and lower motor neurons that causes specific symptoms and clinical signs. The central diagnostic criterion for ALS is the combined degeneration of upper motor neurons of the corticospinal tract and lower motor neurons in the brainstem nuclei and spinal anterior horns.1 However, postmortem studies have revealed that the pathology spreads beyond the motor system in some ALS patients.2 It is known that ALS is a neurodegenerative disorder involving other extramotor areas including the cerebellum,3 as well as primary and secondary motor neurons, and thus causes frontal dysfunction with a clinicopathological overlap with frontotemporal dementia,4 and progressive supranuclear palsy.56
Unlike other motor systems, the ocular motor system was generally believed to be spared in ALS, with the exception of some complicated patients. However, several abnormal findings across diverse eye movements such as fixation,78 saccades,29101112 and smooth pursuits,1012131415 ophthalmoplegia,16 and nystagmus17 can be seen in assessments of oculomotor function. These abnormal eye movements in ALS may provide insight into the pattern and pathogenesis of the disease. Nevertheless, the pattern of oculomotor dysfunction in ALS has not been established.
In this study we aimed to identify the pattern of abnormal oculomotor functions in ALS. We also investigated the characteristics of abnormal oculomotor functions in subgroups of ALS.
A retrospective analysis of clinical and neurophysiological data from patients who attended the authors' clinic for ALS was conducted at Asan Medical Center from April 2009 to August 2011. Patients who fulfilled the Awaji criteria for ALS18 and underwent video-oculography were included. The presence of motor neuron disease was confirmed by detailed history-taking, clinical examinations, and nerve conduction and electromyography studies. Subjects were categorized according to onset pattern into bulbar onset or spinal onset. Patients who presented with both patterns were categorized as bulbar onset.
The present study was reviewed and approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (IRB No. 2014-0482), and adhered to the tenets of the Declaration of Helsinki. Given the retrospective nature of the study and the use of anonymized patient data, the requirement for obtaining informed consent from the subjects was waived.
Eye movements were recorded monocularly using infrared video-oculography (SLMED, Seoul, Korea), which records up to ±30° in the horizontal and vertical planes. All patients underwent a standardized evaluation by a single skilled examiner. The equipment was calibrated before the test with the subject wearing the video-oculography goggles in a seated position. Spontaneous nystagmus and primary gaze fixation were recorded both with and without fixation for 30 seconds. Gaze-evoked nystagmus (GEN) and eccentric gaze fixation was induced with horizontal (±15°) target displacements. Smooth pursuit was studied using a red laser spot that moved horizontally with a constant-velocity waveform (±15°). These tests were performed at frequencies of 0.1, 0.2, and 0.4 Hz in increasing order. Reflexive saccades were evaluated when subjects were requested to follow the red laser spot that was moved between nine positions along a horizontal line between ±15° at random times and positions. Head-shaking nystagmus (HSN) was assessed using a passive head-shaking maneuver. The examiner pitched the patient's head forward by approximately 30° to bring the horizontal semicircular canals into the plane of stimulation. The patient's head was then grasped firmly with both hands and shaken horizontally in a sinusoidal fashion at a rate of 2–3 Hz with an approximate amplitude of ±10° for 10–15 seconds. To induce positional nystagmus, the patients turned their heads to both sides while supine (head turning test), and the Dix-Hallpike maneuver was performed bilaterally.
Abnormal oculomotor functions were confirmed by two neurologists (BH.K. and JI.K.) who reviewed the video recordings and oculography findings via visual analysis. Abnormalities were considered to be present in smooth pursuits and saccades only when consistent patterns were observed during the examination. Saccadic hypometria was diagnosed when the subjects experienced at least three corrective saccades consistently during the saccade test. HSN and positional nystagmus were considered to be present when the mean slow-phase velocity of the nystagmus was ≥3°/s and when the nystagmus lasted >5 s.19
The Mann-Whitney U test and Fisher's exact test of nonparametric statistics were applied to continuous and categorical variables, respectively. The statistical analysis was performed using SPSS (version 18.0, SPSS Inc., Chicago, IL, USA), with p<0.05 considered to be statistically significant.
Five of the 37 consecutive patients with motor neuron disease were excluded due to 3 exhibiting only lower motor neuron signs and being suspicious for progressive muscular atrophy or spinal muscular atrophy, while the other 2 exhibited only upper motor neuron signs and were considered as having primary lateral sclerosis. The remaining 32 patients (13 female and 19 male) fulfilled the Awaji criteria for ALS: 24 were clinically definite, 4 were clinically probable, and 4 were clinically possible.18 One of the clinically definite ALS patients had accompanying parkinsonism. There were 22 spinal-onset and 10 bulbar-onset patients. The subjects in the present study included individuals in the third (n=2), fourth (n=6), fifth (n=8), seventh (n=13), and eighth (n=3) decades of life. Their median age was 59 years (interquartile range=16 years) at the time of the video-oculography examination. The median disease duration was 13.5 months (interquartile range=12.25 months). The mean age was lower in the spinalonset group than in the bulbar-onset group (p=0.035). A summary of the clinical data and abnormal oculomotor findings of the subjects is presented in Table 1.
None of the subjects had a medical history of acute vertigo, visual disturbance, or ear problems. All of the patients were alert and responsive during testing, and none of them exhibited clinical evidence of respiratory failure that required artificial ventilation. In addition, none of the patients had either ophthalmoplegia or spontaneous nystagmus/GEN. They did not have specific structural abnormalities on their brain or cervical spine magnetic resonance imaging. One patient had normal brain computed tomography findings, and MRIs of three patients were not confirmed directly due to deficiencies in the medical records. Fifteen subjects were taking small doses of benzodiazepine and/or baclofen. There were no medical records documenting side effects of these drugs, and the drug use did not differ significantly between the two groups.
Seven (22%) patients exhibited square-wave jerks (one with bulbar onset and six with spinal onset) (Fig. 1A). Fourteen (44%) patients exhibited abnormal smooth pursuit at 0.1 Hz (7 with bulbar onset and 7 with spinal onset), 17 (53%) at 0.2 Hz (9 with bulbar onset and 8 with spinal onset), and 18 (56%) at 0.4 Hz (9 with bulbar onset and 9 with spinal onset). The pursuit defects were present in both directions and similar in appearance with cogwheeling (Fig. 1B), and they were observed in the third (n=2), fourth (n=1), fifth (n=4), seventh (n=9), and eighth (n=2) decades of life. There were 11 (34%) patients with hypometria (7 with bulbar onset and 4 with spinal onset), and 2 patients with hypermetria in the spinal-onset group (1 in each of the fourth and eighth decades of life). Two patients with saccadic dysmetria had unilateral abnormalities, while the others had bilateral abnormalities (Fig. 1C). Saccadic dysmetria was identified in the third (n=1), fourth (n=1), fifth (n=1), seventh (n=7), and eighth (n=3) decades of life. The disease duration did not differ significantly between the bulbar- and spinal-onset groups. Abnormal smooth pursuit and saccadic dysmetria were increased in the bulbar-onset group compared with the spinalonset group (p<0.05) (Table 2).
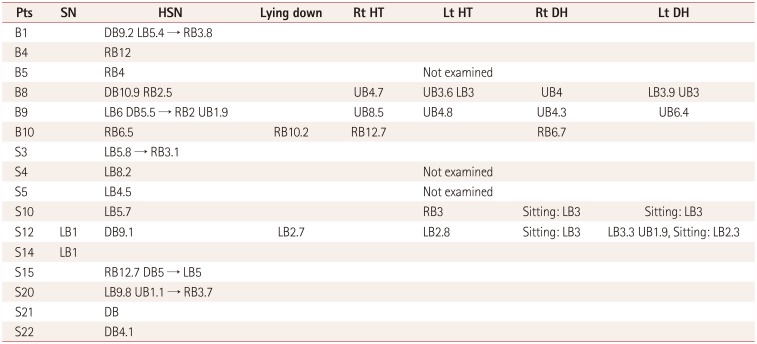
Spontaneous nystagmus with fixation was not identified in the video-oculography results, similarly to the findings of the bedside ocular examinations. Spontaneous nystagmus without fixation revealed left-beating nystagmus with a mean slow-phase velocity of 1°/s in two (6%) patients, and GEN was not found. Seventeen (53%) patients exhibited HSN, including perverted downbeat nystagmus (Supplementary Video 1 in the online-only Data Supplement) and direction-changing nystagmus (Supplementary Video 2 in the onlineonly Data Supplement). The patterns of HSN are specifically described in Table 3 and 4. Twenty-five patients underwent the head-turning and Dix-Hallpike tests. Positional nystagmus was present in five (16%) patients. The nystagmus patterns are delineated in detail in Table 4.
This study found various types of oculomotor dysfunction such as square-wave jerks, saccadic dysmetria, abnormal cogwheeling smooth pursuit, and head shaking, and positional nystagmus in ALS patients at a relatively early disease stage. A particularly interesting finding was that spontaneous nystagmus with fixation and GEN were not recorded in all of the ALS patients. If maneuvers that induce nystagmus (e.g., head shaking and positional changes) were not performed on purpose, the nystagmus would have gone unnoticed. Because some oculomotor functions are often not tested in routine bedside examinations, these might not have been recognized.
Most instances of nystagmus in our subjects were regarded as being central in origin, such as perverted HSN, direction-changing nystagmus, and atypical positional nystagmus. To our knowledge this is the first systematic study of nystagmus in ALS patients. Nystagmus occurs when there is a disturbance within any of the mechanisms that have evolved to hold gaze steady, such as the vestibulo-ocular reflex (VOR). Although the nystagmus patterns of central disorders vary and the mechanisms remain to be elucidated, perverted HSN is explained by abnormal cross-coupling of the velocity storage system or a buildup of vertical vestibular asymmetry favoring upward bias due to disinhibition of the flocculus over the anterior-canal VOR pathway in brainstem and cerebellar lesions.1920 Perverted HSN has been reported previously in diffuse cerebellar degeneration and focal medullary19 or cerebellar lesions.20 Although direction-changing nystagmus in the head-shaking test can be due to severe unilateral peripheral vestibulopathy, it is generally considered to reflect central vestibular dysfunction. Our patients had no histories of acute vertigo or ear symptoms, which makes it less likely that they had peripheral vestibulopathy.
Five of the patients in this study had positional nystagmus. The clinical findings and the patterns of positional nystagmus were not compatible with those of benign paroxysmal positional vertigo (BPPV). For example, positional up-beating nystagmus during the supine roll test (head-turning test) without vertigo in patients B8 and B9 is typical of nystagmus of central origin. These two patients also exhibited perverted and direction-changing nystagmus in the head-shaking test (Table 4), and so their nystagmus may have been caused by central vestibulopathy. The concrete aspects for differentiating from peripheral positional nystagmus are that these cases were not associated with vertigo clinically and the various nystagmus patterns were unexplainable by BPPV (i.e., the direction of nystagmus for the stimulated canal was atypical).21 Although the pathomechanism and clinical characteristics of central positional vertigo/nystagmus remain unclear, it can be caused by a cerebellar dorsal vermis lesion, tumors or hemorrhage in the dorsolateral region of the fourth ventricle, degenerative disorders involving the cerebellum, and sometimes migraine.22 It has been postulated from previous case series and animal studies that the mechanism of central positional nystagmus involves the interruption of cerebellar modulation of gravitational pathways by lesions in the dorsal vermis or near the vestibular nucleus.2122
If spontaneous nystagmus is observed without fixation and disappears after fixation, it is commonly associated with the subacute or chronic course of peripheral nystagmus. This situation was observed in two patients in the present study (Table 4). However, considering that the clinical history was less likely to be due to peripheral vestibulopathy and patient S12 had perverted (down-beating) HSN, central vestibulopathy was initially considered. The other patient (patient S14) had only spontaneous nystagmus without fixation. It was therefore difficult to identify the cause, and there is also the possibility of it having been normal.
Head-shaking and positional stimuli can induce nystagmus in normal subjects that is transient, mostly lasting less than several seconds. Although the result was not directly compared with that of normal controls in this study, there was high probability of definitive findings because of the strict criteria used to identify nystagmus. Nystagmus observed in ALS patients may be attributable to lesions in central vestibular pathways including the vestibulocerebellum; however, the precise localization remains to be determined.
Defective smooth pursuit movements such as reduced velocity gain or abnormal catch-up saccades were commonly described in ALS in comparison with normal controls,2101114 Smooth pursuit is controlled by cerebro-ponto-cerebellar pathways, and the neural substrate comprises both cortical and subcortical components. The neural substrate of fixation and the conditions of saccadic intrusions are quite similar to those of smooth pursuit. Several studies have described fixation abnormalities in ALS patients. For example, the saccadic intrusion amplitude was found to be higher in ALS patients than in controls and correlated with neuropsychological measures sensitive to lesions of the frontal lobes.7 Unfortunately we could not evaluate age-matched normal controls and the gains in smooth pursuit or the latencies and velocities of saccades quantitatively due to limitations of the employed video-oculography apparatus. Our ALS patients were observed to exhibit frequent abnormal smooth pursuit even in low speeds, and frequent square-wave jerks even at relatively young ages.
Abnormalities of saccadic accuracy (especially hypermetria) are usually considered to indicate cerebellar and brainstem dysfunctions. Also saccadic dysmetria has occasionally been reported in patients with peripheral ocular motor disorders, such as myasthenia gravis, and reflects an adaptive response to a primary motor deficit.23 Although our patients did not exhibit clinically definite ophthalmoplegia, it is unclear whether their abnormal saccadic accuracy was due to cerebellar dysfunction, dysfunction of the ocular motor neurons themselves, or a compensatory central adaptation to a primary motor deficit.
Abnormal smooth pursuits and saccadic dysmetria were more common in the bulbar-onset group than the spinal-onset group in this study. This finding is cautiously interpreted as indicating that central pathology is likely to be more extensive in bulbar-onset patients than in spinal-onset patients, making damage in the areas known to cause abnormal smooth pursuit and saccades more likely in the former group. Future longitudinal studies with quantitative measurements of eye-movement abnormalities should be needed to verify it.
This study had several limitations. First, it is possible that our results were somewhat exaggerated, especially regarding smooth pursuit and square-wave jerks, because these features were not compared with normal controls. Second, insufficient evaluations were applied to the peripheral vestibular system, such as head impulse and caloric tests. Third, the central dysfunctions could have been better explained if visual fixation test and recordings of torsional eye movement had been included in this study. Finally, ALS was diagnosed clinically in this study, and several subjects who were in the early stages of disease were included. Follow-up examinations and longitudinal studies could help to address these concerns.
Our observational study has demonstrated that oculomotor dysfunction and nystagmus are observed in ALS. Perverted HSN and central positional nystagmus in our ALS patients could be due to dysfunction of vestibulocerebellar connections. Oculomotor abnormalities may be a marker of neurodegeneration beyond motor neurons in ALS, especially in bulbar-onset disease. Further longitudinal studies and precise localizations are needed. Our data may provide useful insights into the distribution and nature of the ALS.
References
1. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the world federation of neurology research group on neuromuscular diseases and the El Escorial “clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994; 124(Suppl):96–107. PMID: 7807156.
2. Averbuch-Heller L, Helmchen C, Horn AK, Leigh RJ, Buttner-Ennerver JA. Slow vertical saccades in motor neuron disease: correlation of structure and function. Ann Neurol. 1998; 44:641–648. PMID: 9778263.

3. Prell T, Grosskreutz J. The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013; 14:507–515. PMID: 23889583.

4. Talbot PR, Goulding PJ, Lloyd JJ, Snowden JS, Neary D, Testa HJ. Inter-relation between “classic” motor neuron disease and frontotemporal dementia: neuropsychological and single photon emission computed tomography study. J Neurol Neurosurg Psychiatry. 1995; 58:541–547. PMID: 7745399.

5. McCluskey LF, Elman LB, Martinez-Lage M, Van Deerlin V, Yuan W, Clay D, et al. Amyotrophic lateral sclerosis-plus syndrome with TAR DNA-binding protein-43 pathology. Arch Neurol. 2009; 66:121–124. PMID: 19139310.

6. Donaghy C, Thurtell MJ, Pioro EP, Gibson JM, Leigh RJ. Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. J Neurol Neurosurg Psychiatry. 2011; 82:110–116. PMID: 21097546.

7. Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Ocular fixation instabilities in motor neurone disease. a marker of frontal lobe dysfunction? J Neurol. 2009; 256:420–426. PMID: 19306041.
8. Palmowski A, Jost WH, Prudlo J, Osterhage J, Kasmann B, Schimrigk K, et al. Eye movement in amyotrophic lateral sclerosis: a longitudinal study. Ger J Ophthalmol. 1995; 4:355–362. PMID: 8751101.
9. Moon SY, Lee BH, Seo SW, Kang SJ, Na DL. Slow vertical saccades in the frontotemporal dementia with motor neuron disease. J Neurol. 2008; 255:1337–1343. PMID: 18825435.

10. Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Slow saccades in bulbar-onset motor neurone disease. J Neurol. 2010; 257:1134–1140. PMID: 20146069.

11. Leveille A, Kiernan J, Goodwin JA, Antel J. Eye movements in amyotrophic lateral sclerosis. Arch Neurol. 1982; 39:684–686. PMID: 7125995.

12. Ohki M, Kanayama R, Nakamura T, Okuyama T, Kimura Y, Koike Y. Ocular abnormalities in amyotrophic lateral sclerosis. Acta Otolaryngol Suppl. 1994; 511:138–142. PMID: 8203216.

13. Abel LA, Williams IM, Gibson KL, Levi L. Effects of stimulus velocity and acceleration on smooth pursuit in motor neuron disease. J Neurol. 1995; 242:419–424. PMID: 7595671.

14. Jacobs L, Bozian D, Heffner RR Jr, Barron SA. An eye movement disorder in amyotrophic lateral sclerosis. Neurology. 1981; 31:1282–1287. PMID: 7202138.

15. Moss HE, McCluskey L, Elman L, Hoskins K, Talman L, Grossman M, et al. Cross-sectional evaluation of clinical neuro-ophthalmic abnormalities in an amyotrophic lateral sclerosis population. J Neurol Sci. 2012; 314:97–101. PMID: 22192877.

16. Mizutani T, Aki M, Shiozawa R, Unakami M, Nozawa T, Yajima K, et al. Development of ophthalmoplegia in amyotrophic lateral sclerosis during long-term use of respirators. J Neurol Sci. 1990; 99:311–319. PMID: 2086731.

17. Kushner MJ, Parrish M, Burke A, Behrens M, Hays AP, Frame B, et al. Nystagmus in motor neuron disease: clinicopathological study of two cases. Ann Neurol. 1984; 16:71–77. PMID: 6465863.

18. Carvalho MD, Swash M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph Lateral Scler. 2009; 10:53–57. PMID: 18985466.

19. Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction: patterns and possible mechanisms. Neurology. 2007; 68:1337–1344. PMID: 17452577.

20. Huh YE, Kim JS. Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain. 2011; 134:3662–3671. PMID: 22036958.

21. Choi JY, Kim JH, Kim HJ, Glasauer S, Kim JS. Central paroxysmal positional nystagmus: characteristics and possible mechanisms. Neurology. 2015; 84:2238–2246. PMID: 25957336.

22. Lee TK. [Central positional vertigo]. Res Vestib Sci. 2011; 10:19–23.
23. Schmidt D, Dell'Osso LF, Abel LA, Daroff RB. Myasthenia gravis: saccadic eye movement waveforms. Exp Neurol. 1980; 68:346–364. PMID: 7363999.

SUPPLEMENTARY MATERIALS
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.4.464.
Fig. 1
Examples of abnormal oculomotor functions in our patients with amyotrophic lateral sclerosis. A: Square-wave jerks, B: Abnormal smooth pursuits, and C: Saccadic hypometria and hypermetria.
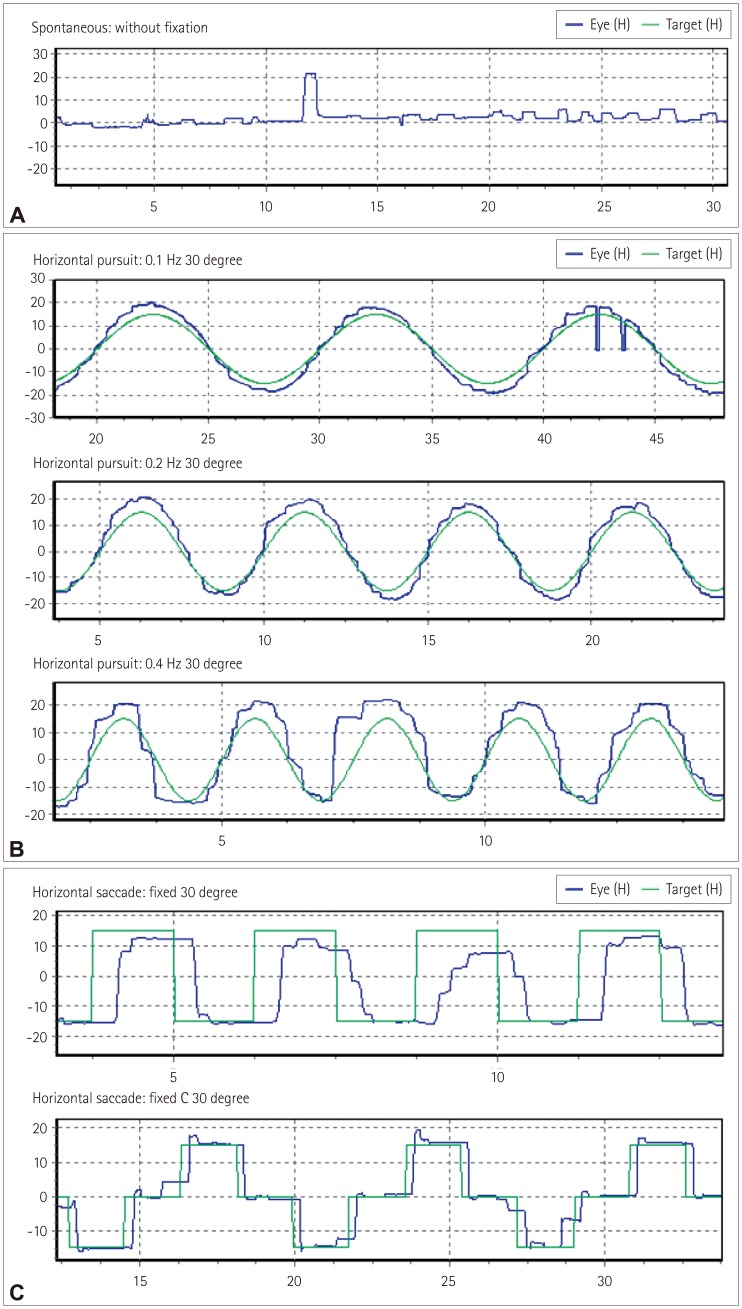
Table 1
Clinical data and types of oculomotor dysfunction of the amyotrophic lateral sclerosis subjects
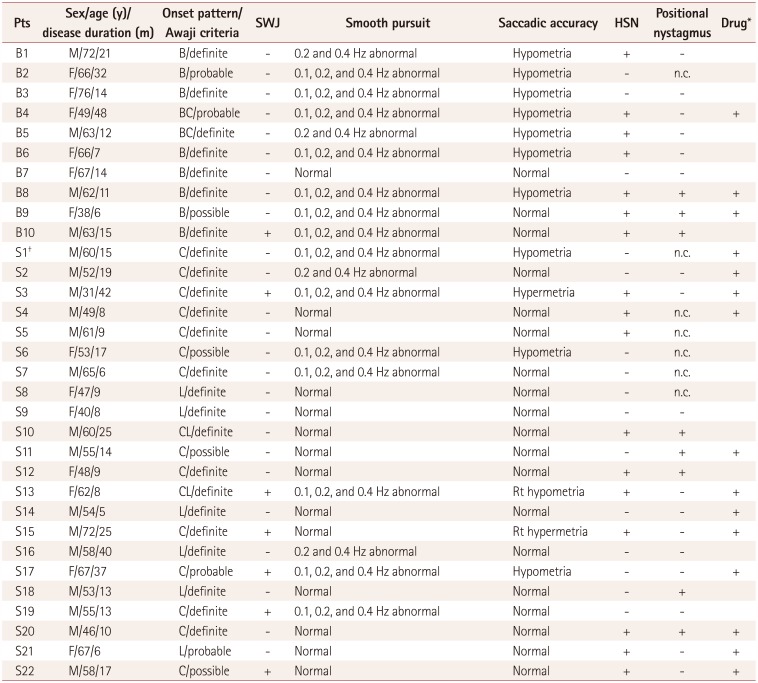
Table 2
Comparison of oculomotor dysfunction between the bulbar-onset and spinal-onset amyotrophic lateral sclerosis patients
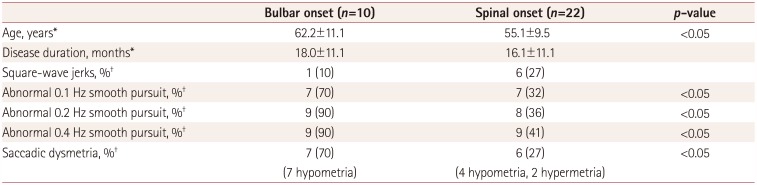
Table 3
Occurrence of nystagmus in the amyotrophic lateral sclerosis patients
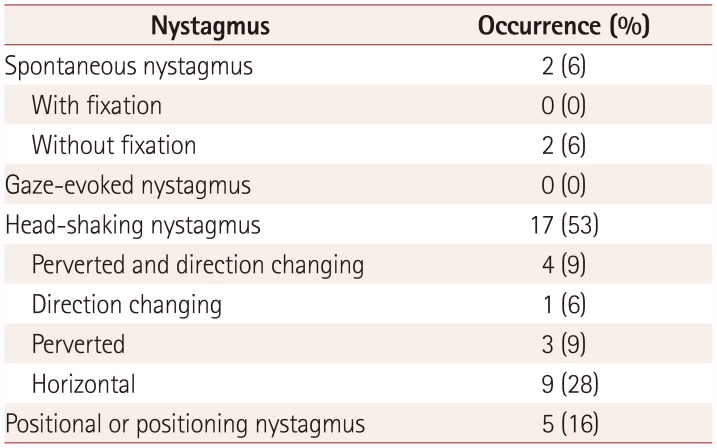
Table 4
Nystagmus patterns in the amyotrophic lateral sclerosis patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download