Abstract
References
Table 1
Demographic characteristics
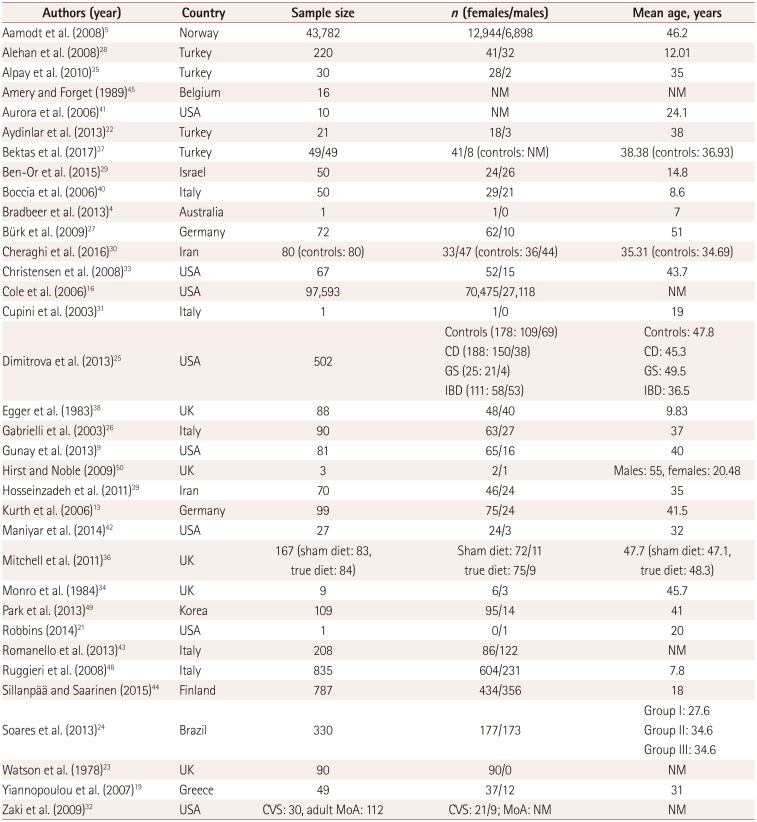
| Authors (year) | Country | Sample size | n (females/males) | Mean age, years |
|---|---|---|---|---|
| Aamodt et al. (2008)5 | Norway | 43,782 | 12,944/6,898 | 46.2 |
| Alehan et al. (2008)28 | Turkey | 220 | 41/32 | 12.01 |
| Alpay et al. (2010)35 | Turkey | 30 | 28/2 | 35 |
| Amery and Forget (1989)45 | Belgium | 16 | NM | NM |
| Aurora et al. (2006)41 | USA | 10 | NM | 24.1 |
| Aydinlar et al. (2013)22 | Turkey | 21 | 18/3 | 38 |
| Bektas et al. (2017)37 | Turkey | 49/49 | 41/8 (controls: NM) | 38.38 (controls: 36.93) |
| Ben-Or et al. (2015)29 | Israel | 50 | 24/26 | 14.8 |
| Boccia et al. (2006)40 | Italy | 50 | 29/21 | 8.6 |
| Bradbeer et al. (2013)4 | Australia | 1 | 1/0 | 7 |
| Bürk et al. (2009)27 | Germany | 72 | 62/10 | 51 |
| Cheraghi et al. (2016)30 | Iran | 80 (controls: 80) | 33/47 (controls: 36/44) | 35.31 (controls: 34.69) |
| Christensen et al. (2008)33 | USA | 67 | 52/15 | 43.7 |
| Cole et al. (2006)16 | USA | 97,593 | 70,475/27,118 | NM |
| Cupini et al. (2003)31 | Italy | 1 | 1/0 | 19 |
| Dimitrova et al. (2013)25 | USA | 502 |
Controls (178: 109/69) CD (188: 150/38) GS (25: 21/4) IBD (111: 58/53) |
Controls: 47.8 CD: 45.3 GS: 49.5 IBD: 36.5 |
| Egger et al. (1983)38 | UK | 88 | 48/40 | 9.83 |
| Gabrielli et al. (2003)26 | Italy | 90 | 63/27 | 37 |
| Gunay et al. (2013)9 | USA | 81 | 65/16 | 40 |
| Hirst and Noble (2009)50 | UK | 3 | 2/1 | Males: 55, females: 20.48 |
| Hosseinzadeh et al. (2011)39 | Iran | 70 | 46/24 | 35 |
| Kurth et al. (2006)13 | Germany | 99 | 75/24 | 41.5 |
| Maniyar et al. (2014)42 | USA | 27 | 24/3 | 32 |
| Mitchell et al. (2011)36 | UK | 167 (sham diet: 83, true diet: 84) |
Sham diet: 72/11 true diet: 75/9 |
47.7 (sham diet: 47.1, true diet: 48.3) |
| Monro et al. (1984)34 | UK | 9 | 6/3 | 45.7 |
| Park et al. (2013)49 | Korea | 109 | 95/14 | 41 |
| Robbins (2014)21 | USA | 1 | 0/1 | 20 |
| Romanello et al. (2013)43 | Italy | 208 | 86/122 | NM |
| Ruggieri et al. (2008)48 | Italy | 835 | 604/231 | 7.8 |
| Sillanpää and Saarinen (2015)44 | Finland | 787 | 434/356 | 18 |
| Soares et al. (2013)24 | Brazil | 330 | 177/173 |
Group I: 27.6 Group II: 34.6 Group III: 34.6 |
| Watson et al. (1978)23 | UK | 90 | 90/0 | NM |
| Yiannopoulou et al. (2007)19 | Greece | 49 | 37/12 | 31 |
| Zaki et al. (2009)32 | USA | CVS: 30, adult MoA: 112 | CVS: 21/9; MoA: NM | NM |
Table 2
Study design
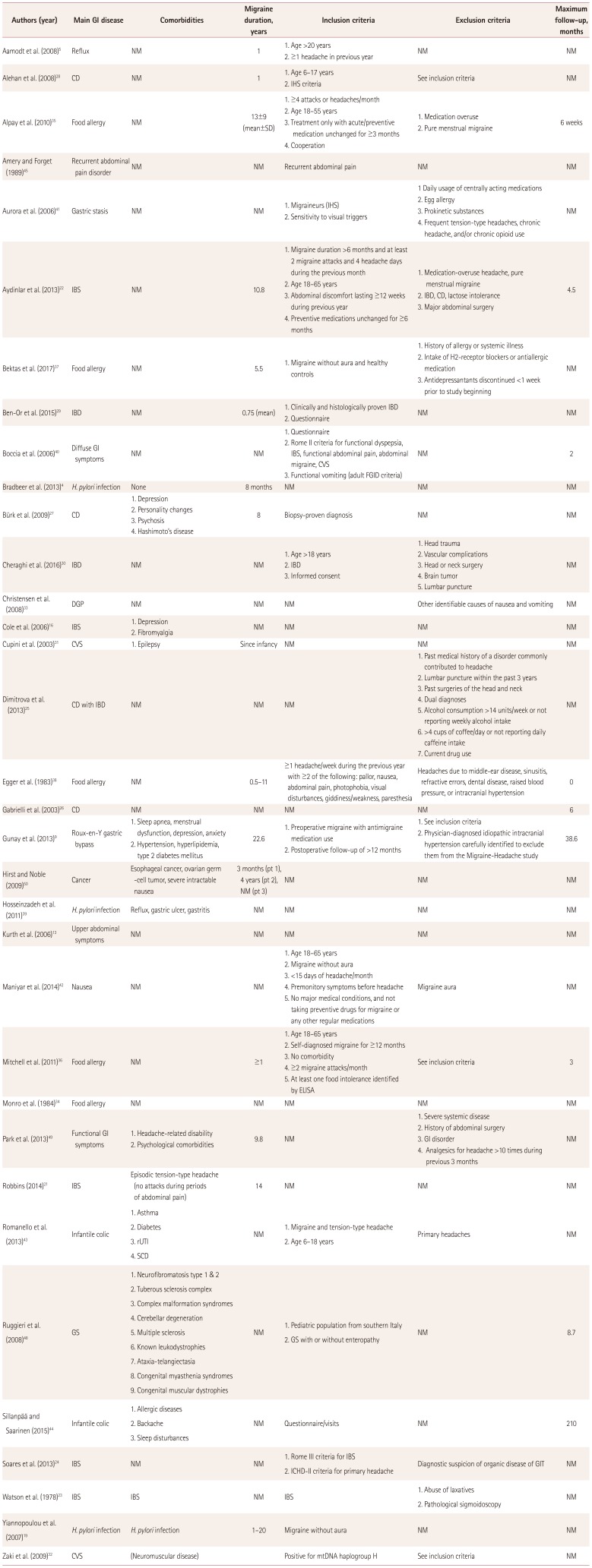
| Authors (year) | Main GI disease | Comorbidities | Migraine duration, years | Inclusion criteria | Exclusion criteria | Maximum follow-up, months |
|---|---|---|---|---|---|---|
| Aamodt et al. (2008)5 | Reflux | NM | 1 |
1. Age >20 years 2. ≥1 headache in previous year |
NM | NM |
| Alehan et al. (2008)28 | CD | NM | 1 |
1. Age 6–17 years 2. IHS criteria |
See inclusion criteria | NM |
| Alpay et al. (2010)35 | Food allergy | NM | 13±9 (mean±SD) |
1. ≥4 attacks or headaches/month 2. Age 18–55 years 3. Treatment only with acute/preventive medication unchanged for ≥3 months 4. Cooperation |
1. Medication overuse 2. Pure menstrual migraine |
6 weeks |
| Amery and Forget (1989)45 | Recurrent abdominal pain disorder | NM | NM | Recurrent abdominal pain | NM | NM |
| Aurora et al. (2006)41 | Gastric stasis | NM | NM |
1. Migraineurs (IHS) 2. Sensitivity to visual triggers |
1 Daily usage of centrally acting medications 2. Egg allergy 3. Prokinetic substances 4. Frequent tension-type headaches, chronic headache, and/or chronic opioid use |
NM |
| Aydinlar et al. (2013)22 | IBS | NM | 10.8 |
1. Migraine duration >6 months and at least 2 migraine attacks and 4 headache days during the previous month 2. Age 18–65 years 3. Abdominal discomfort lasting ≥12 weeks during previous year 4. Preventive medications unchanged for ≥6 months |
1. Medication-overuse headache, pure menstrual migraine 2. IBD, CD, lactose intolerance 3. Major abdominal surgery |
4.5 |
| Bektas et al. (2017)37 | Food allergy | NM | 5.5 | 1. Migraine without aura and healthy controls |
1. History of allergy or systemic illness 2. Intake of H2-receptor blockers or antiallergic medication 3. Antidepressantants discontinued <1 week prior to study beginning |
NM |
| Ben-Or et al. (2015)29 | IBD | NM | 0.75 (mean) |
1. Clinically and histologically proven IBD 2. Questionnaire |
NM | NM |
| Boccia et al. (2006)40 | Diffuse GI symptoms | NM | NM |
1. Questionnaire 2. Rome II criteria for functional dyspepsia, IBS, functional abdominal pain, abdominal migraine, CVS 3. Functional vomiting (adult FGID criteria) |
NM | 2 |
| Bradbeer et al. (2013)4 | H. pylori infection | None | 8 months | NM | NM | NM |
| Bürk et al. (2009)27 | CD |
1. Depression 2. Personality changes 3. Psychosis 4. Hashimoto’s disease |
8 | Biopsy-proven diagnosis | NM | NM |
| Cheraghi et al. (2016)30 | IBD | NM | NM |
1. Age >18 years 2. IBD 3. Informed consent |
1. Head trauma 2. Vascular complications 3. Head or neck surgery 4. Brain tumor 5. Lumbar puncture |
NM |
| Christensen et al. (2008)33 | DGP | NM | NM | NM | Other identifiable causes of nausea and vomiting | NM |
| Cole et al. (2006)16 | IBS |
1. Depression 2. Fibromyalgia |
NM | NM | NM | NM |
| Cupini et al. (2003)31 | CVS | 1. Epilepsy | Since infancy | NM | NM | NM |
| Dimitrova et al. (2013)25 | CD with IBD | NM | NM | NM |
1. Past medical history of a disorder commonly contributed to headache 2. Lumbar puncture within the past 3 years 3. Past surgeries of the head and neck 4. Dual diagnoses 5. Alcohol consumption >14 units/week or not reporting weekly alcohol intake 6. >4 cups of coffee/day or not reporting daily caffeine intake 7. Current drug use |
NM |
| Egger et al. (1983)38 | Food allergy | NM | 0.5–11 | ≥1 headache/week during the previous year with ≥2 of the following: pallor, nausea, abdominal pain, photophobia, visual disturbances, giddiness/weakness, paresthesia | Headaches due to middle-ear disease, sinusitis, refractive errors, dental disease, raised blood pressure, or intracranial hypertension | 0 |
| Gabrielli et al. (2003)26 | CD | NM | NM | NM | NM | 6 |
| Gunay et al. (2013)9 | Roux-en-Y gastric bypass |
1. Sleep apnea, menstrual dysfunction, depression, anxiety 2. Hypertension, hyperlipidemia, type 2 diabetes mellitus |
22.6 |
1. Preoperative migraine with antimigraine medication use 2. Postoperative follow-up of >12 months |
1. See inclusion criteria 2. Physician-diagnosed idiopathic intracranial hypertension carefully identified to exclude them from the Migraine-Headache study |
38.6 |
| Hirst and Noble (2009)50 | Cancer | Esophageal cancer, ovarian germ-cell tumor, severe intractable nausea | 3 months (pt 1), 4 years (pt 2), NM (pt 3) | NM | NM | NM |
| Hosseinzadeh et al. (2011)39 | H. pylori infection | Reflux, gastric ulcer, gastritis | NM | NM | NM | NM |
| Kurth et al. (2006)13 | Upper abdominal symptoms | NM | NM | NM | NM | NM |
| Maniyar et al. (2014)42 | Nausea | NM | NM | 1. Age 18–65 years 2. Migraine without aura 3. <15 days of headache/month 4. Premonitory symptoms before headache 5. No major medical conditions, and not taking preventive drugs for migraine or any other regular medications | Migraine aura | NM |
| Mitchell et al. (2011)36 | Food allergy | NM | ≥1 | 1. Age 18–65 years 2. Self-diagnosed migraine for ≥12 months 3. No comorbidity 4. ≥2 migraine attacks/month 5. At least one food intolerance identified by ELISA | See inclusion criteria | 3 |
| Monro et al. (1984)34 | Food allergy | NM | NM | NM | NM | NM |
| Park et al. (2013)49 | Functional GI symptoms |
1. Headache-related disability 2. Psychological comorbidities |
9.8 | NM |
1. Severe systemic disease 2. History of abdominal surgery 3. GI disorder 4. Analgesics for headache >10 times during previous 3 months |
NM |
| Robbins (2014)21 | IBS | Episodic tension-type headache (no attacks during periods of abdominal pain) | 14 | NM | NM | NM |
| Romanello et al. (2013)43 | Infantile colic |
1. Asthma 2. Diabetes 3. rUTI 4. SCD |
NM |
1. Migraine and tension-type headache 2. Age 6–18 years |
Primary headaches | NM |
| Ruggieri et al. (2008)48 | GS |
1. Neurofibromatosis type 1 & 2 2. Tuberous sclerosis complex 3. Complex malformation syndromes 4. Cerebellar degeneration 5. Multiple sclerosis 6. Known leukodystrophies 7. Ataxia-telangiectasia 8. Congenital myasthenia syndromes 9. Congenital muscular dystrophies |
NM |
1. Pediatric population from southern Italy 2. GS with or without enteropathy |
NM | 8.7 |
| Sillanpää and Saarinen (2015)44 | Infantile colic | 1. Allergic diseases 2. Backache 3. Sleep disturbances | NM | Questionnaire/visits | NM | 210 |
| Soares et al. (2013)24 | IBS | NM | NM |
1. Rome III criteria for IBS 2. ICHD-II criteria for primary headache |
Diagnostic suspicion of organic disease of GIT | NM |
| Watson et al. (1978)23 | IBS | IBS | NM | IBS | 1. Abuse of laxatives 2. Pathological sigmoidoscopy | NM |
| Yiannopoulou et al. (2007)19 | H. pylori infection | H. pylori infection | 1–20 | Migraine without aura | NM | NM |
| Zaki et al. (2009)32 | CVS | (Neuromuscular disease) | Positive for mtDNA haplogroup H | See inclusion criteria | NM |
CD: celiac disease, CVS: cyclic vomitings syndrome, DGP: diabetic gastropathy, ELISA: enzyme linked immunosorbent assay, FGID: functional gastrointestinal disorders, GI: gastrointestinal, GIT: gastrointestinal tract, GS: gluten sensitivity, H. pylori: Helicobacter pylori, IBD: inflamatory bowel disease, IBS: irritable bowel syndrome, ICHD: International Classification of Headache Disorders, IHS: International Headache Society, NM: not mentioned, pt: patient, rUTI: recurrent urinary tract infection, SCD: Sickle-cell disease.
Table 3
GI manifestations and possible pathogenetic mechanisms underlying the correlation between the CNS and GID
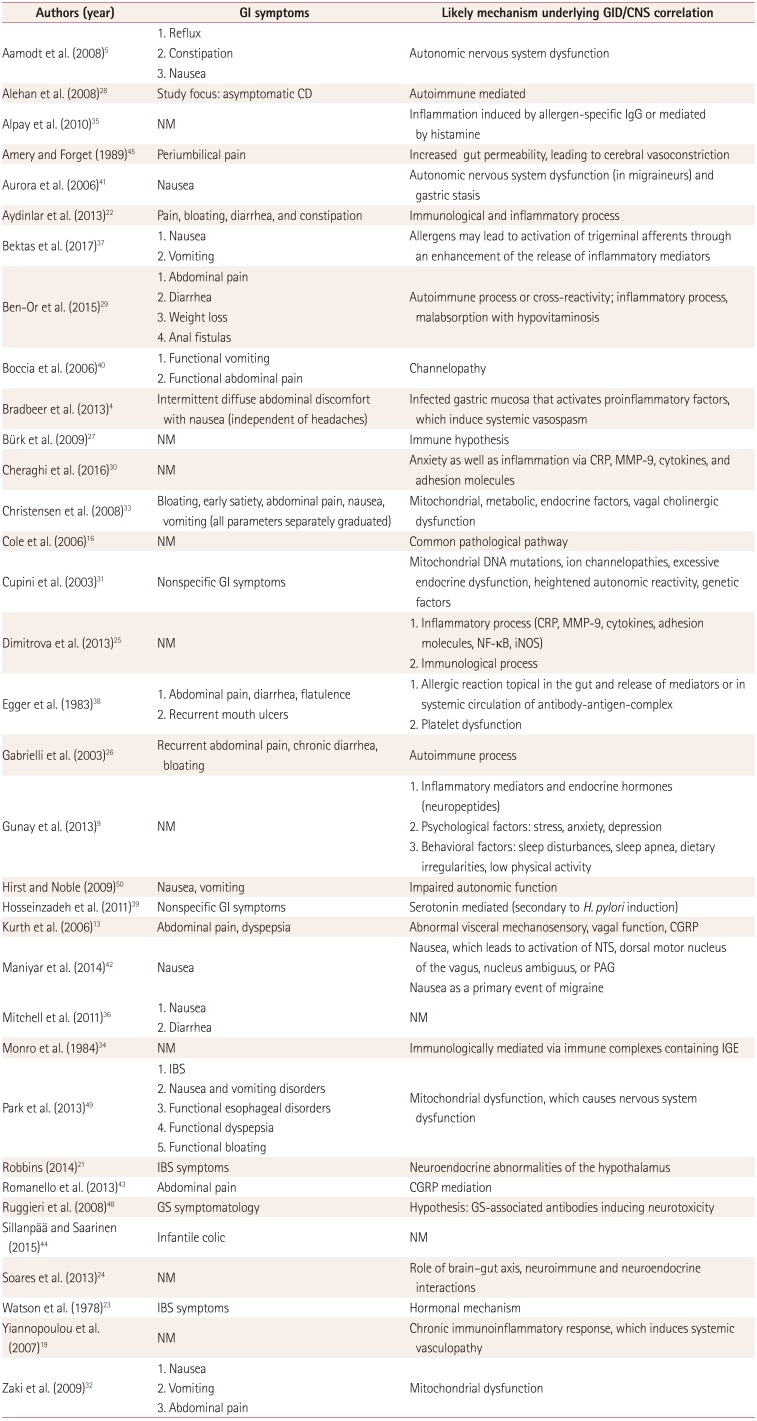
| Authors (year) | GI symptoms | Likely mechanism underlying GID/CNS correlation |
|---|---|---|
| Aamodt et al. (2008)5 |
1. Reflux 2. Constipation 3. Nausea |
Autonomic nervous system dysfunction |
| Alehan et al. (2008)28 | Study focus: asymptomatic CD | Autoimmune mediated |
| Alpay et al. (2010)35 | NM | Inflammation induced by allergen-specific IgG or mediated by histamine |
| Amery and Forget (1989)45 | Periumbilical pain | Increased gut permeability, leading to cerebral vasoconstriction |
| Aurora et al. (2006)41 | Nausea | Autonomic nervous system dysfunction (in migraineurs) and gastric stasis |
| Aydinlar et al. (2013)22 | Pain, bloating, diarrhea, and constipation | Immunological and inflammatory process |
| Bektas et al. (2017)37 |
1. Nausea 2. Vomiting |
Allergens may lead to activation of trigeminal afferents through an enhancement of the release of inflammatory mediators |
| Ben-Or et al. (2015)29 |
1. Abdominal pain 2. Diarrhea 3. Weight loss 4. Anal fistulas |
Autoimmune process or cross-reactivity; inflammatory process, malabsorption with hypovitaminosis |
| Boccia et al. (2006)40 |
1. Functional vomiting 2. Functional abdominal pain |
Channelopathy |
| Bradbeer et al. (2013)4 | Intermittent diffuse abdominal discomfort with nausea (independent of headaches) | Infected gastric mucosa that activates proinflammatory factors, which induce systemic vasospasm |
| Bürk et al. (2009)27 | NM | Immune hypothesis |
| Cheraghi et al. (2016)30 | NM | Anxiety as well as inflammation via CRP, MMP-9, cytokines, and adhesion molecules |
| Christensen et al. (2008)33 | Bloating, early satiety, abdominal pain, nausea, vomiting (all parameters separately graduated) | Mitochondrial, metabolic, endocrine factors, vagal cholinergic dysfunction |
| Cole et al. (2006)16 | NM | Common pathological pathway |
| Cupini et al. (2003)31 | Nonspecific GI symptoms | Mitochondrial DNA mutations, ion channelopathies, excessive endocrine dysfunction, heightened autonomic reactivity, genetic factors |
| Dimitrova et al. (2013)25 | NM |
1. Inflammatory process (CRP, MMP-9, cytokines, adhesion molecules, NF-κB, iNOS) 2. Immunological process |
| Egger et al. (1983)38 |
1. Abdominal pain, diarrhea, flatulence 2. Recurrent mouth ulcers |
1. Allergic reaction topical in the gut and release of mediators or in systemic circulation of antibody-antigen-complex 2. Platelet dysfunction |
| Gabrielli et al. (2003)26 | Recurrent abdominal pain, chronic diarrhea, bloating | Autoimmune process |
| Gunay et al. (2013)9 | NM |
1. Inflammatory mediators and endocrine hormones (neuropeptides) 2. Psychological factors: stress, anxiety, depression 3. Behavioral factors: sleep disturbances, sleep apnea, dietary irregularities, low physical activity |
| Hirst and Noble (2009)50 | Nausea, vomiting | Impaired autonomic function |
| Hosseinzadeh et al. (2011)39 | Nonspecific GI symptoms | Serotonin mediated (secondary to H. pylori induction) |
| Kurth et al. (2006)13 | Abdominal pain, dyspepsia | Abnormal visceral mechanosensory, vagal function, CGRP |
| Maniyar et al. (2014)42 | Nausea |
Nausea, which leads to activation of NTS, dorsal motor nucleus of the vagus, nucleus ambiguus, or PAG Nausea as a primary event of migraine |
| Mitchell et al. (2011)36 |
1. Nausea 2. Diarrhea |
NM |
| Monro et al. (1984)34 | NM | Immunologically mediated via immune complexes containing IGE |
| Park et al. (2013)49 |
1. IBS 2. Nausea and vomiting disorders 3. Functional esophageal disorders 4. Functional dyspepsia 5. Functional bloating |
Mitochondrial dysfunction, which causes nervous system dysfunction |
| Robbins (2014)21 | IBS symptoms | Neuroendocrine abnormalities of the hypothalamus |
| Romanello et al. (2013)43 | Abdominal pain | CGRP mediation |
| Ruggieri et al. (2008)48 | GS symptomatology | Hypothesis: GS-associated antibodies inducing neurotoxicity |
| Sillanpää and Saarinen (2015)44 | Infantile colic | NM |
| Soares et al. (2013)24 | NM | Role of brain–gut axis, neuroimmune and neuroendocrine interactions |
| Watson et al. (1978)23 | IBS symptoms | Hormonal mechanism |
| Yiannopoulou et al. (2007)19 | NM | Chronic immunoinflammatory response, which induces systemic vasculopathy |
| Zaki et al. (2009)32 |
1. Nausea 2. Vomiting 3. Abdominal pain |
Mitochondrial dysfunction |
CD: celiac disease, CGRP: calcitonin-gene-related peptide, CNS: central nervous system, CRP: C-reactive protein, GI: gastrointestinal, GID: gastrointestinal disorders, GS: gluten sensitivity, H. pylori: Helicobacter pylori, IBS: irritable bowel syndrome, IGE: Immunoglobulin E, iNOS: inducible nitric oxide synthase, MMP-9: matrix metallopeptidase-9, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, NM: not mentioned, NTS: nucleus tractus solitarius, PAG: periaqueductal gray.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download