Dear Editor,
Ataxia with oculomotor apraxia type I (AOA1) is a recessively inherited ataxic disorder that is characterized clinically by the childhood onset of progressive cerebellar ataxia, oculomotor apraxia (OMA), and peripheral axonal sensorimotor neuropathy.1 Dystonia, chorea, and cognitive impairment are commonly associated symptoms, and hypoalbuminemia and hypercholesterolemia are often observed.2 AOA1 is caused by mutations of the gene encoding aprataxin (APTX).3 AOA1 has not been reported previously in Korea.5
The first case of AOA1 in Korea is presented here. In this patient, who did not exhibit OMA but had bilateral gaze-evoked nystagmus, the condition was caused by compound heterozygous mutations of APTX.
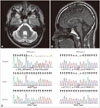
A 32-year-old man presented with slowly progressive gait disturbance and unsteadiness that developed at the age of 14 years. He was the second child of healthy unrelated Korean parents without a family history of gait disturbance. He was diagnosed as having cerebellar atrophy of unknown cause at the age of 17 years. His ataxic gait had progressively deteriorated and he had become confined to a wheelchair 2 years prior to presentation. A physical examination revealed pes cavus but no scoliosis or other musculoskeletal deformity. A neurological examination revealed mild cerebellar dysarthria without dysphagia. His limb motor powers were normal, as were light touch, pinprick, temperature, and pain sensations. However, his vibration and positional senses were decreased in the lower limbs, his deep tendon reflexes were hyporeflexic in all the limbs, Babinski signs were absent bilaterally, and he had bilateral upper and lower limb dysmetria and truncal ataxia. The patient was unable to stand still without assistance. His ranges of extraocular movement were full in all directions, and he did not exhibit OMA on reflexive saccades testing, but his optokinetic nystagmus was impaired. He presented gaze-evoked nystagmus in all directions. His Mini-Mental State Examination score was 30. The results of laboratory tests were unremarkable except for hypercholesterolemia (217 mg/dL), hypoalbuminemia (3.3 g/dL), and mildly elevated alpha-fetoprotein (7.05 ng/mL). A nerve conduction study and electromyography revealed sensorimotor axonal polyneuropathy, and brain magnetic resonance imaging revealed pure cerebellar atrophy without involvement of the pons, medulla oblongata, midbrain, or cerebral cortex (Fig. 1A and B). Sanger sequencing after PCR amplification of APTX revealed compound heterozygous mutations involving the deletion of two nucleotides (c.359_360delAC, p.Asp120Lysfs2) in exon 3, and a missense mutation (c.617C>T, p.Pro206Leu, rs121908131) in exon 5 (Fig. 1C). While the mutation of c.359_360delAC is novel, c.617C>T is a known pathogenic mutation. The patient's father is a carrier of the single heterozygous mutation (c.617C>T); we were unable to perform genetic testing on his mother.
While Friedreich's ataxia is the most frequent cause of autosomal recessive ataxia in the Caucasian population,6 it is rare in Korea and Japan.57 Before discovery of the APTX mutation, AOA1 was known as early-onset ataxia with OMA and hypoalbuminemia (EAOH) in Japan (MIM 208920).3 EAOH is reportedly the most common cause of autosomal recessive hereditary ataxia in Japan.48 To the best of our knowledge, this is the first case of AOA1 with a confirmed APTX mutation in Korea.5 Hereditary ataxia with OMA is genetically heterogeneous; to date, four genes (APTX, SETX, PIK3R5, and PNKP) have been found to cause AOA syndromes (AOA1, AOA2, AOA3, and AOA4, respectively). Decreased deep tendon reflexes and peripheral neuropathy are not infrequently found with hereditary ataxia. However, elevated alpha-fetoprotein levels, hypoalbuminemia, and hypercholesterolemia are observed in only some of the ataxic disorders. The alpha-fetoprotein level is typically elevated in ataxia-telangiectasia, AOA2, and spinocerebellar ataxia with axonal neuropathy (SCAN1), and but is uncommon in AOA1, AOA3, and AOA4.9 We could make a clinical diagnosis of AOA syndrome based on laboratory features.
The most common presenting symptom of AOA1 is gait disturbance. Abnormal eye movement or head thrust is recognized as an initial symptom in fewer than 10% of cases. Although gaze-evoked nystagmus is reportedly present in more than 70% of AOA1 cases,2 it has limited diagnostic value since gaze-evoked nystagmus in all directions occurs in many neurodegenerative disorders associated with impaired function of the flocculus/paraflocculus.10 It has been reported that OMA was not present in 34.5% of AOA1 cases, and that it may ultimately progress to external ophthalmoplegia.2 Given that our patient had an 18-year history of gait unsteadiness, and that cerebellar atrophy was documented 15 years prior to this presentation, the absence of OMA in this patient is unusual, although impaired optokinetic nystagmus suggests early voluntary OMA. OMA can be observed in other hereditary ataxic disorders such as ataxia-telangiectasia, ataxia-telangiectasia-like disorder 1, autosomal recessive spinocerebellar ataxias 5 and 18, autosomal recessive spastic ataxia 5, Joubert syndrome, COACH syndrome, pyruvate dehydrogenase E2 deficiency, and orofaciodigital syndrome VI.
In summary, this case indicates that AOA1 should be included in the differential diagnosis of early-onset ataxia in Korea, even in the absence of OMA.
Figures and Tables
Fig. 1
Brain MRI and Sanger sequencing of APTX exons. A and B: Coronal T2-weighted (A) and sagittal T1-weighted (B) magnetic resonance images of the proband. Note the presence of pure cerebellar atrophy without involvement of the pons, medulla oblongata, or cerebral cortex. C: Electropherogram showing mutations in exons 3 and 5 of APTX. Mutation spots of c.359_360delAC (p.Asp120Lysfs2) and c.617C>T (p.Pro206Leu, rs121908131) are marked by a red bar.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A4A01007783).
References
1. Coutinho P, Barbot C. Ataxia with Oculomotor Apraxia Type 1. In : Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, editors. SourceGeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle;1993-2015.
2. Yokoseki A, Ishihara T, Koyama A, Shiga A, Yamada M, Suzuki C, et al. Genotype-phenotype correlations in early onset ataxia with ocular motor apraxia and hypoalbuminaemia. Brain. 2011; 134(Pt 5):1387–1399.

3. Date H, Onodera O, Tanaka H, Iwabuchi K, Uekawa K, Igarashi S, et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat Genet. 2001; 29:184–188.

4. Shimazaki H, Takiyama Y, Sakoe K, Ikeguchi K, Niijima K, Kaneko J, et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia: the aprataxin gene mutations. Neurology. 2002; 59:590–595.

5. Kim JS, Cho JW. Hereditary cerebellar ataxias: a Korean perspective. J Mov Disord. 2015; 8:67–75.

6. Anheim M, Fleury M, Monga B, Laugel V, Chaigne D, Rodier G, et al. Epidemiological, clinical, paraclinical and molecular study of a cohort of 102 patients affected with autosomal recessive progressive cerebellar ataxia from Alsace, Eastern France: implications for clinical management. Neurogenetics. 2010; 11:1–12.

7. Sasaki H, Yabe I, Yamashita I, Tashiro K. Prevalence of triplet repeat expansion in ataxia patients from Hokkaido, the northernmost island of Japan. J Neurol Sci. 2000; 175:45–51.

8. Tsuji S. [Clinical features and molecular genetics of autosomal recessive spinocerebellar degenerations]. Rinsho Shinkeigaku. 2004; 44:785–787.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download