This article has been corrected. See "ERRATUM: Citicoline Protects Against Cognitive Impairment in a Rat Model of Chronic Cerebral Hypoperfusion" in Volume 5 on page 104.
Abstract
Background and purpose
Cerebral white matter (WM) lesions are frequently observed in human cerebrovascular diseases, and are believed to be responsible for cognitive impairment. Various neuroprotective agents can suppress this type of WM or neuronal damage. In this study, we investigated whether citicoline, a drug used to treat acute ischemic stroke, can attenuate WM lesions and cognitive decline caused by chronic hypoperfusion in the rat.
Methods
Animals were divided into immediate- and delayed-treatment groups. Those in the immediate-treatment group received a sham operation, citicoline (500 mg/kg/day), or phosphate buffered saline (PBS) treatment. Citicoline or PBS was administered intraperitoneally for 21 days after occluding the bilateral common carotid arteries. Rats in the delayed-treatment group were intraperitoneally administered with either 500 mg/kg/day citicoline or PBS for 21 days beginning on the 8th day after the operation. From the 17th day of administration, the rats were placed in an eight-arm radial maze to examine their cognitive abilities. After completing the administration, tissues were isolated for Klüver-Barrera and the terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) staining.
Results
In the immediate-treatment group, cognitive functions were preserved in the citicoline-treated group, and WM damage and TUNEL-positive cells differed significantly between the citicoline- and PBS-treated animals. In the delayed-treatment group, there was no decrease in WM damage and TUNEL-positive cells, but cognitive improvement was evident for citicoline treatment relative to PBS treatment.
Vascular dementia is a subtype of dementia, and its prevalence is second to that of Alzheimer's disease in westernized societies.1 Vascular dementia causes many neuropsychiatric and physical problems, and represents a significant economic burden. Brain imaging studies have revealed obvious changes in the cerebral white matter (WM) rather than in the cortex, and hence WM lesions are thought to be the core pathology for cognitive declines in patients with vascular dementia.2,3 It is known that WM damage is caused by widespread demyelination and axonal loss in the WM;4 however, the mechanisms underlying the pathologic changes are not fully understood.
Cerebral WM lesions are probably caused by chronic cerebral ischemia, and they can indeed be experimentally induced in the rat brain by permanent occlusion of both common carotid arteries,5 which can affect cognitive function,6 and this model is similar to that of vascular dementia.7 This technique can decrease the blood flow in the cerebral cortex and hippocampus by up to 40-82%8-10 for several months, which induces certain learning disorders. Thus, this technique can be used to study vascular dementia and cerebral WM lesions.
It has been reported that citicoline (exogenous cytidine-5-diphosphocholine), a substance synthesized by Eugene Kennedy in 1956, is effective in treating ischemia in the central nervous system, brain trauma, and Parkinson's disease.11 Citicoline is a precursor of phosphatidylcholine (PtdCho) biosynthesis, and an increase in PtdCho biosynthesis prevents apoptosis of neurons and protects against neuronal damage.12 In addition, citicoline maintains cardiolipin and sphingomyelin levels, stimulates glutathione biosynthesis, activates glutathione reductase, and reduces lipid peroxidation.12 Therefore, citicoline is expected to prevent apoptosis and delay disease progression in the brains of rats with chronic ischemia.
In the present study, we investigated the therapeutic effects of citicoline in chronic cerebral hypoperfusion induced in rats by conducting a histopathologic investigation and cognitive-behavior test.
All procedures used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at the College of Medicine, The Catholic University of Korea. Ten-week-old male Wistar rats weighing 280-300 g (Joongang Lab, Seoul, Korea) were used in this study. The rats were housed at a temperature of 23-26℃ and a humidity of 50% with a 12-h light-dark cycle, and they were allowed free access to food and water except when performing the eight-arm radial maze.
All surgical procedures were carried out under inhalation anesthesia using 2% isofluorane with 30% O2 and 70% N2, and the body temperature was maintained during the operation at 37.0-37.8℃ using a temperature controller. After fixing a rat in the prone position on a board, a skin incision about 2 cm long was made and a cervical central line was placed. The bilateral common carotid arteries were exposed by isolating them from the vagus nerve and its sheath, and they were double ligated with 4-0 silk at 8-10 mm below the visible region of the external carotid artery.
Animals were divided into immediate- and delayed-treatment groups. Those in the immediate-treatment group received a sham operation (n=8), citicoline (Somazina, Bukwang, Korea) administered intraperitoneally (500 mg/kg/day) for 3 weeks immediately after the operation (n=9), or phosphate buffered saline (PBS)(n=7).
Rats in the delayed-treatment group were administered intraperitoneally with 500 mg/kg/day citicoline (n=8) or PBS (n=8) for 21 days beginning on the 8th day after the operation.
Rats in the immediate-treatment group were given 500 mg/kg/day citicoline for 3 weeks beginning immediately after the operation.13 PBS was administered at the same dosage in control animals. In addition, the chronic effects of citicoline on the progression of vascular change were examined in the delayed-treatment group.
The spatial memory test was performed in an eight-arm radial maze comprising a center with a radius of 34 cm and eight arms (60 cm long and 12 cm wide) with walls 20 cm high. Rats were trained twice daily for 10 days before model production, and rats with less than two total errors (TEs) were selected for further testing. The rats were then fed ad libitum for 3 hours and then fasted. Four of the eight arms in the maze contained 40-50 mg of feed that was placed in the same place every day. Considering the visual loss after model production, the floor paper in each arm had a different texture in order to allow the rats to identify the arms containing feed.14 The rats were placed in the center of the maze, and a test was carried out for 10 minutes to see if the rats ate all of the feed. A TE, reference memory error (RME), or walking memory error (WME) was considered to have occurred if the rat went to the arm without feed, went to the arm without feed for the first time, or returned to the arm without feed after eating, respectively. The time taken to eat all of the feed was recorded.
Rats were deeply anesthetized with 30% urethane, perfused transcardially with 0.01 M PBS, and then perfused with a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) containing 0.2% glutaraldehyde. The brain was extracted and fixed again with the fixative for 4 hours and soaked in a 30% sucrose solution until it sank. It was then quick-frozen with liquid nitrogen and stored at -70℃. Slices with thicknesses of 25 and 6 µm were then made by cutting at 2.8-3.14 mm posterior to the bregma using a cryomicrotome in accordance with the atlas of Paxinos and Watson.15
Each 25-µm-thick section was soaked in 0.1% Solvent Blue 38 (Sigma, USA) solution at 60℃ overnight, and the dye was removed (except for that in the WM region) using lithium carbonate solution, distilled water, and 70% ethanol. The section was soaked in 0.1% cresyl violet (Sigma) solution for 15 minutes, and then in 95% ethanol followed by 100% ethanol and xylene for dehydration and transparency. After mounting, pathological findings in the optic tract, internal capsule, and corpus callosum were observed using an optical microscope at 200× magnification. The severity of the WM lesion was classified as normal (grade 0), disarrangement of nerve fibers (grade 1), formation of marked vacuoles (grade 2), and disappearance of myelinated fibers (grade 3).16
The terminal deoxynucleotidyl transferase biotin-dUTP nickend-labeling (TUNEL) assay was performed on 6-µm-thick brain sections using fluorescein with the In Situ Cell Death Detection Kit (Roche, Germany). TUNEL-positive cells were then examined using a fluorescent microscope.
The eight-arm radial maze test was carried out for 5 days before sacrificing the animals. In the immediate-treatment group on the first day, the number of TEs did not differ significantly between sham (5.56±1.45) and citicoline (5.05±0.64) treatments but it did differ between PBS treatment (7.21±1.17) and sham and citicoline treatments. The total time spent and number of RMEs were somewhat higher for PBS treatment (338.57±35.24 s and 3.21±0.28, respectively) than for sham (278.88±56.50 s and 2.5±0.39) and citicoline (294.88±34.03 s and 2.66±0.25) treatments. In the last 5 days, there were decreases in TEs, time spent, and RMEs for all treatments: 1.25±0.42, 113.06±16.96 s, and 0.75±0.18, respectively, for sham treatment; 2.61±0.68, 131.06±26.76 s, and 1.27±0.33 for citicoline treatment; and 4.42±1.52, 246.64±49.11 s, and 2.00±0.34 for PBS treatment (Fig. 1). No significant difference was found in the frequency of WMEs, but the frequency was too low to determine the effect of citicoline on short-term memory disorder.
In the delayed-treatment group, the number of TEs did not differ significantly between citicoline (6.25±1.34) and PBS (6.31±0.87) treatments on the first day. However, from the second day, the difference in the number of TEs between citicoline and PBS treatments gradually increased over time, eventually becoming significant (at 3.12±0.65 and 4.00±0.64, respectively) on the 5th day. The time spent did not differ between citicoline and PBS treatments on the 1st day (423.81±58.76 and 426.25±47.93 s, respectively) but it did on the 5th day (178.37±37.05 and 223.43±40.80 s). In addition, the frequency of memory errors eventually differed significantly between citicoline treatment (2.87±0.30 and 1.81±0.33 on the 1st and 5th days, respectively) and PBS treatment (3.12±0.24 and 2.68±0.32)(Fig. 2). The frequency of WMEs did not differ significantly in the delayed-treatment group.
Rats in the immediate-treatment group showed marked differences in the optic tract, some differences in the corpus callosum (Fig. 3A and B), and few changes in the internal capsule and other WM regions. The extent of WM changes in the optic tract and corpus callosum of rats was graded according to the standard of Wakita et al.13 as follows: 0.23±0.04, 1.52±0.13, and 1.85±0.15 for sham, citicoline, and PBS treatments, respectively, in the optic tract; and 0.20±0.04, 0.50±0.08, and 0.88±0.17 in the corpus callosum (p<0.05).
In the delayed-treatment group, WM changes were observed in the optic tract and corpus callosum, but there was no significant difference between citicoline and PBS treatments: 2.09±0.09 and 1.98±0.12, respectively, in the optic tract; and 0.83±0.09 and 0.96±0.13 in the corpus callosum (Fig. 3D and E).
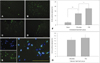
There were TUNEL-positive cells around cortex and lateral ventricles (Fig. 4C and D) as well as in the WM (Fig. 4A and B), but not in the hippocampus.
The number of TUNEL-positive cells that were also stained with DAPI (excluding positive cells in vessels of sections)17 (Fig. 4E) was significantly higher for citicoline (12.66±4.29 cells) and PBS (17.42±6.94 cells) treatments than for the sham treatment (2.62±0.88 cells) in the immediate-treatment group (Fig. 4F). Similar numbers of TUNEL-positive cells were observed in the delayed-treatment group, but there was no significant difference between citicoline and PBS treatments (12.75±1.71 and 14.37±2.42, respectively)(Fig. 4G).
In the immediate-treatment group, WM damage and apoptosis were lower for citicoline treatment than for PBS treatment. Phosphatidylcholine is a cell membrane component that is degraded during cerebral ischemia into free fatty acids and free radicals.18,20 Because citicoline is an intermediate in the synthesis of phosphatidylcholine, it might counter the progression of ischemic damage by reducing the release of free fatty acids18,20 and by recovering activities of mitochondrial ATPase and membrane Na+/K+ ATPase.18 Also, it prevents apoptosis by improving membrane recovery.19 These findings are consistent with our results. Therefore, citicoline improves memory and learning ability by reducing the number of ischemic lesions and exerting neuroprotective effects on ischemia via the above mechanisms.
In the delayed-treatment group, WM lesions and apoptosis did not differ between citicoline and PBS treatments, but there was an improvement in cognitive ability. These results indicate that citicoline acts via a different mechanism in addition to neuroprotection in cognitive improvement. Although the exact mechanism of cognitive improvement is not known, citicoline might prevent cognitive impairment by increasing phospholipids, which have an important role in neurotransmission,21 and by increasing the levels of norepinephrine and dopamine,22 which are known to be neurotransmitters involved in cognition. Further study is needed to elucidate the actual mechanism.
Citicoline is used as an immediate treatment for neuroprotection in patients with acute stroke, but the effects of delayed treatment of citicoline on cognition have been unclear. This study confirms that delayed treatment improves the cognitive decline that is a typical symptom in dementia. Therefore, it is likely that citicoline can be applied more widely in patients with vascular dementia.
Figures and Tables
Fig. 1
Eight-arm radial maze test of immediate treatments with citicoline, PBS, and a sham operation. Number of TEs (A), number of RMEs (B), and time spent (C). †p<0.05 vs citicoline treatment, §p<0.05 vs citicoline treatment, *p<0.05, **p<0.01 vs. sham operation. PBS: phosphate buffered saline, TEs: total error, RMEs: reference memory errors.

Fig. 2
Eight-arm radial maze test of delayed treatments with citicoline, PBS, and a sham operation. Number of TEs (A), number of RMEs (B), time spent (C). *p<0.05, **p<0.01. PBS: phosphate buffered saline, TEs: total error, RMEs: reference memory errors.

Fig. 3
Klüver-Barrera staining for WM damage. Corpus callosum and optic tract of rats that received a sham operation, citicoline, or PBS in the immediate-treatment (A) and delayed-treatment (B) groups. Schematic representation of the rat brain at 3.14 mm posterior to the bregma (C). Grade of Klüver-Barrera staining for WM damage in the corpus callosum and optic tract in the immediate-treatment (D) and delayed-treatment (E) groups. The WM damage was greatest in the optic tract, with less intense changes observed in the medial part of the corpus callosum. Note that citicoline caused less serious WM damage than PBS in both the optic tract and corpus callosum in the immediate-treatment group, whilst there was no significant difference in the delayed-treatment group. Scale bars in A, B=60 µm. *p<0.01, **p<0.001. WM: white matter, PBS: phosphate buffered saline.
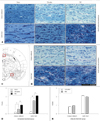
Fig. 4
TUNEL staining in the immediate-treatment group. TUNEL-positive cells were evident both in WM such as the optic tract (A) and internal capsule (B) and also in gray matter such as the infarcted cortex (C) and subventricular zone (D). We only counted positive cells that were also stained with DAPI (E). TUNEL-positive cells in the immediate-treatment (F) and delayed-treatment (G) groups. The number of TUNEL-positive cells was lower for citicoline treatment than for PBS treatment in the immediate-treatment group, whilst there was no significant difference in the delayed-treatment group. Scale bars in A-E=200 µm. *p<0.05, **p<0.001. TUNEL: the terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling, WM: white matter.
Acknowledgments
This Study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and welfare, Republic of Korea (No. A040018).
References
1. Sachdev PS, Brodaty H, Looi JC. Vascular dementia: diagnosis, management and possible prevention. Med J Aust. 1999. 170:81–85.

2. Farkas E, Donka G, de Vos RA, Mihály A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004. 108:57–64.

3. Stenset V, Hofoss D, Berstad AE, Negaard A, Gjerstad L, Fladby T. White matter lesion subtypes and cognitive deficits in patients with memory impairment. Dement Geriatr Cogn Disord. 2008. 26:424–431.

5. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994. 87:484–492.

6. Kim JE, Lee BR, Chun JE, Lee SJ, Lee BH, Yu IK, et al. Cognitive Dysfunction in 16 patients with carotid stenosis: detailed neuropsychological Findings. J Clin Neurol. 2007. 3:9–17.

7. Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience. 2008. 155:626–639.

8. Otori T, Katsumata T, Katayama Y, Terashi A. Measurement of regional cerebral blood flow and glucose utilization in rat brain under chronic hypoperfusion conditions following bilateral carotid artery occlusion. Analyzed by autoradiographical methods. Nippon Ika Daigaku Zasshi. 1997. 64:428–439.

9. Tomimoto H, Akiguchi I, Wakita H, Kimura J. White matter lesions after occlusion of the bilateral carotid arteries in the rat--temporal profile of cerebral blood flow (CBF), oligodendroglia and myelin. No To Shinkei. 1997. 49:639–644.
10. Tsuchiya M, Sako K, Yura S, Yonemasu Y. Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res. 1992. 89:87–92.

11. Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002. 70:133–139.

12. Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002. 80:12–23.

13. Hurtado O, Moro MA, Cárdenas A, Sánchez V, Fernández-Tomé P, Leza JC, et al. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport. Neurobiol Dis. 2005. 18:336–345.

14. Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000. 859:96–103.

15. Paxinos G, Watson C. The rat brain in stereotaxic coodinates. 1998. 4th ed. San Diego: Academic press.
16. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res. 1998. 792:105–113.

17. Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, et al. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003. 106:527–534.

18. Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation? J Neurol Sci. 2007. 257:264–269.

19. Onal MZ, Li F, Tatlisumak T, Locke KW, Sandage BW Jr, Fisher M. Synergistic effects of citicoline and MK-801 in temporary experimental focal ischemia in rats. Stroke. 1997. 28:1060–1065.

20. Paul RH, Cohen RA, Moser DJ, Ott BR, Sethi M, Sweet L, et al. Clinical correlates of cognitive decline in vascular dementia. Cogn Behav Neurol. 2003. 16:40–46.

21. Agut J, Lopez G-Coviella I, Ortiz JA, Wurtman RJ. Oral cytidine 5'-diphosphate choline administration to rats increases brain phospholipid levels. Ann N Y Acad Sci. 1993. 695:318–320.

22. Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006. 28:Suppl B. 1–56.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download