Abstract
Background and purpose
Congenital nystagmus (CN) is an ocular oscillation that usually manifests during early infancy. Typical features of CN include bilateral, conjugate, uniplanar, and usually horizontal eye movements, a null position, increased oscillation during fixation, and decreased amplitude during convergence. Our purposes were description and analysis of clinical and oculomotor findings of patients with X-linked familial CN.
Methods
We describe the clinical and oculographic features of five patients from three families with X-linked CN. Three-dimensional video-oculography disclosed various patterns of CN and variable degrees of gaze-holding deficits and visual impairments.
Congenital nystagmus (CN) is characterized by bilateral ocular oscillations with an onset typically during infancy.1 Typical features of CN include bilateral, conjugate, uniplanar, and usually horizontal eye movements, a null position, increased oscillation during fixation, and decreased amplitude during convergence.1 It should be differentiated from nystagmus associated with other ocular disorders, such as albinism, optic nerve hypoplasia, retinal diseases, foveal hypoplasia, early visual deprivation, perinatal insults, and lesions of the central nervous system resulting in cortical visual impairment.2-4 Visual acuity in CN depends on many factors, including developmental mechanisms, afferent visual abnormalities, and variations in the foveation time.5
Variable patterns of inheritance (X-linked, autosomal dominant, and autosomal recessive) have been described in CN.2,3,6-10 However, X-linkage with variable penetrance appears to be the most common mode of inheritance in the few mapping studies that have been performed.7 We report on the clinical and oculographic characteristics of CN in five patients from three Korean pedigrees with presumed X-linked inheritance.
Five patients with CN from three families underwent full neuro-otological and neuro-ophthalmological evaluations at the Neuro-Ophthalmology Clinic of Seoul National University Bundang Hospital from March to September 2005. The criteria used to diagnose CN were (1) a history of nystagmus from infancy, (2) bilateral conjugate ocular oscillations, and (3) no abnormalities in the afferent visual pathways. Affected family members were determined based on a history of nystagmus with an onset within the first 6 months of life without other visual or neurological abnormalities other than strabismus. Informed consents were obtained from all participants after the methods and possible consequences of this study had been explained.
The presence of nystagmus was determined both with and without fixation using video Frenzel goggles (SLMED, Seoul, Korea). The effects of fixation, gaze, convergence, and optokinetic stimuli were evaluated. In all patients, eye movements were recorded by three-dimensional video-oculography (SMI, Teltow, Germany).11 We tested for spontaneous nystagmus with and without fixation, gaze-evoked nystagmus (GEN) in the horizontal (±30°) and vertical (±20°) planes, vibration-induced nystagmus, head-shaking nystagmus (HSN), and positional nystagmus. Positional nystagmus was assessed during serial position changes, which included sitting, lying down supine, turning the head to both sides while supine, straight head hanging, and both Hallpike maneuvers.12
HSN was assessed using the passive head-shaking maneuver. Briefly, whilst holding the patient's head firmly on both sides, the head was tilted forward about 30° and then moved sideways in a sinusoidal fashion at a rate of 2~3 Hz for 15 s. On completion, the patient was instructed to open his/her eyes and to look straight ahead. Recordings were continued until HSN resolved.11
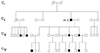
A 6-year-old boy was found to have spontaneous pendular eye movements shortly after birth, and leftward head tilt during the first 3 years of life (Fig. 1). Horizontal, conjugate, and pendular nystagmus was predominant in the primary position (Fig. 2-a), and was suppressed with removal of the fixation (Fig. 2-b) or with convergence. The nystagmus changed into jerky nystagmus during lateral gaze. Left-beating, upbeating, and counterclockwise (CCW) torsional nystagmus appeared during leftward gaze (Fig. 2-c), while rightbeating, upbeating, and clockwise (CW) torsional nystagmus appeared during rightward gaze. The direction of optokinetic nystagmus (OKN) was reversed. These continuous eye movements did not cause oscillopsia, reading difficulty, or dizziness. The uncorrected visual acuity was 0.3 in the right eye and 0.5 in the left eye. Gaze limitation or latent nystagmus was not observed. The visual field and color vision were intact. Several members of his family had abnormal ocular oscillations (Fig. 1).
The 39-year-old mother of patient 1 was found to have abnormal eye movements and rightward head tilt when she was 1 year old. The patient also later suffered from thyroid cancer and diabetes. On examination, she showed right exotropia and nystagmus with left-beating, downbeating, and CCW torsional components in the primary position (Fig. 3-a). The nystagmus was right-beating, upbeating, and CW torsional during rightward gaze (Fig. 3-b), and left-beating, downbeating, and CCW torsional during leftward gaze (Fig. 3-c). The nystagmus was mildly suppressed during vertical gaze. The OKN was reversed. Her uncorrected visual acuity was 0.4 in both eyes. She claimed that dizziness and oscillopsia were absent.
A 23-year-old man suffered from continuous ocular oscillations and oscillopsia that his parents had detected during infancy. Oscillopsia was accentuated during fixation and during upward and bilateral gaze. He had difficulty reading books and watching TV. On examination, his head was tilted to the right and his face was turned to the left. He also showed continuous horizontal pendular nystagmus (Fig. 4-a), which changed to right- and left-beating horizontal jerky nystagmus during rightward (Fig. 4-b) and leftward gazes. During vertical gaze, the pendular nystagmus continued with suppression. Convergence also reduced the nystagmus (Fig. 4-c).
The 20-year-old brother of patient 3 (Fig. 5) showed involuntary eye movements that had been detected by his parents during infancy. His general physical findings were normal. He showed spontaneous left-beating horizontal nystagmus (Fig. 6-a) that was accentuated during leftward gaze. The nystagmus changed into subtle right-beating during rightward gaze. Nystagmus was suppressed during vertical gaze and convergence. Removal of fixation changed the direction of nystagmus into right beating (Fig. 6-b), and the OKN was reversed (Fig. 6-c). His visual acuity was 0.2 in the right eye and 1.2 in the left eye, and he had 50 prism diopters of right exotropia. The patient claimed that diplopia and dizziness were absent, and had no complaints or difficulties related to the nystagmus.
A 75-year-old man (Fig. 7) visited our clinic for the evaluation of strabismus and involuntary eye movements that had been detected by his parents during infancy. He claimed that diplopia and dizziness were absent. However, abnormal ocular oscillation significantly interfered with his vision. His general physical findings were normal. He had a visual acuity of 0.2 in the right eye and 0.4 in the left eye, and showed subtle exotropia in the left eye. Spontaneous left-beating nystagmus was observed during attempted forward gaze. His color vision was normal. Other members of his family over three generations had similar ocular oscillations (Fig. 7). Recordings showed predominantly left-beating nystagmus with a small rotatory component (Fig. 8-a). Without fixation, the direction of spontaneous left-beating nystagmus changed to the right with occasional null points (Fig. 8-b).
The clinical features of the five patients are summarized in Table 1. The onset of nystagmus was within the first 2 years of life in all of them. Some family members of the patients experienced fixation difficulty, oscillopsia, mild dizziness, or loss of balance. These symptoms were not characterized by paroxysmal exacerbations or progressive deterioration, and no neuroimaging abnormalities were identified.
The visual acuities ranged from 0.2 to 0.8 in our five patients, with a median of 0.4. Vision was relatively well preserved and color vision was normal in all cases. Exotropia was present in two patients. No other neurological or systemic disorders were present in any of the patients.
The eye-movement abnormalities were characterized by pendular or jerky oscillations, GEN, and poor or absent smooth pursuit and vestibulo-ocular reflex (VOR; Table 2). The nystagmus waveforms were pendular, jerky, or a combination thereof. The patients also showed typical features of CN in various combinations, including increased velocity waveforms, foveation periods, direction change with gaze shift, and reversed OKN. The patterns of nystagmus differed even among patients from the same family. In most patients, the nystagmus had a null position in which the amplitude was reduced. Without fixation, the nystagmus decreased in two patients and changed its direction in another two patients. All patients showed reduced nystagmus during convergence, reversed OKN, and impaired saccades and smooth pursuits. Head shaking induced downbeating nystagmus in one patient but had no affect on CN in the remaining patients. Positional maneuvers had no effect on the direction or intensity of spontaneous nystagmus in most of the patients. However, one patient (patient 3) exhibited downbeating nystagmus for several seconds during straight head hanging.
The pedigrees of our patients indicate an X-linked pattern of inheritance without father-to-son transmission. The presence of generation skipping and more male than female offspring also suggest X-linked inheritance with variable penetrance. Among the three families, penetrance among obligate female carriers was 49.7% (pedigree A, 50%; pedigree B, 46%; and pedigree C, 53%).
We have summarized the clinical features and oculographical findings in three families of X-linked CN. Affected family members had typical features of CN. Transillumination defects were absent, color vision was normal, and the optic nerves were not hypoplastic. All examined patients showed normally developed foveae.
Oculography revealed variable nystagmus waveforms even in patients from the same family, which suggests that heredity does not play a major role in determining CN waveforms. The most distinctive feature of CN is its waveforms,13 with the most common being an increasing slow phase velocity and pendular waveforms. Our patients also showed either jerky or pendular waveforms, GEN, and changes in the amplitude and shape of the waveform as the gaze moved away from the center. Direction reversal with gaze shift is a common finding in CN.1 GEN, absence or very poor performance of smooth pursuits, and saccadic and VOR abnormalities were frequently observed in our patients (Table 2). Four patients (patients 1.4) demonstrated horizontal gaze-holding deficits, indicating a leaky neural integrator. GEN and poor smooth pursuit are observed in a wide range of cerebellar disorders and may also reflect drug intoxication. Moreover, these eye-movement abnormalities can be induced by lesions in the flocculus and paraflocculus of the vestibulocerebellum. Given that the functional role of the flocculus is to keep retinal images of stationary or moving objects stable by adaptive eye-movement control, gaze-holding failure is a putative cause of CN.14,15
The variation in visual acuity in our patients suggests that factors other than CN itself play a role in determining vision. These may include genes or nongenetic developmental mechanisms, such as amblyopia or variations in the development of fixation. The intrafamilial variability in visual acuity that we observed applies to binocular vision. However, the eye-movement records of our patients showed that visual acuity depends upon the accuracy and duration of target fixation. Fixation was more stable in those with good visual acuity (patients 1, 2, and 4) than in those with poor visual acuity (patients 3 and 5), who tended to have poor foveal fixation periods. Poor vision was strongly associated with the nystagmus intensity and the frequency of null periods. More visual detail is obtained when the eyes remain longer on a target, with full visual acuity being achieved if images are present for at least 100 ms at the fovea.14,15 This appears to explain the correlations between visual acuity and frequency of foveation periods.
Several patterns of CN inheritance have been described previously, including X-linked, autosomal recessive, and autosomal dominant.2,3,6-10 However, the inheritance patterns cannot be distinguished clinically using the visual acuity or the nystagmus waveforms. The notable feature of the pedigrees of our patients was the absence of male-to-male transmission. If the genetics of our patients followed an autosomal dominant pattern with variable penetrance, there would probably have been examples of an affected male with an affected son. The absence of such a case among our pedigrees suggests that the X chromosome is responsible, since the absence of male-to-male penetrance is regarded as a hallmark of Xlinkage. We also found no typical features of autosomal dominant inheritance, such as all generations being affected or the absence of transmission from unaffected members. Therefore, our pedigrees indicated the presence of X-linked inheritance with variable penetrance. The reasons for incomplete penetrance among female carriers may include skewed X inactivation, interactions with other genes, and nongenetic developmental influences on ocular motor development. 16,17
Diverse phenotypes of a single abnormal gene tend to preclude accurate etiological classifications based entirely on clinical signs.17 Our patients from the same family exhibited diverse nystagmus waveforms. The coexistence of pendular and jerky in two of our patients (patients 1 and 3) also suggests that unknown variables are involved in the ocular motor control systems of the affected individuals.
The present examination of multiple affected persons within three pedigrees of X-linked CN revealed diverse CN features, even in patients from the same family. The intra- and interfamilial diversities imply that heredity plays a secondary role in determining the clinical phenotypes and waveforms of CN.
Figures and Tables
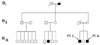
Figure 1
Pedigree of family A: solid symbols (circles=females, squares=males) indicate clinically affected individuals; open symbols, unaffected individuals; and slashed symbols, deceased individuals. The affection status of the deceased individuals was obtained historically. Generation: I; first, II; second, III; third, and IV; fourth.
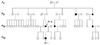
Figure 2
(a) Spontaneous nystagmus with fixation. The horizontal conjugate pendular nystagmus was predominant in the primary position. (b) Spontaneous nystagmus without fixation. The nystagmus was suppressed mildly when the fixation was removed. (c) Left-beating, upbeating, and counterclockwise (CCW) torsional nystagmus appears during leftward gaze. The slow phase is an increased velocity pattern (LH; left eye horizontal, LV; left eye vertical, RH; right eye horizontal, RV; right eye vertical, vel; velocity). Upward deflection in this and the following figures indicates rightward, upward, and clockwise (CW) eye motion.
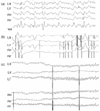
Figure 3
Oculography of patient 2. (a) Left-beating, downbeating, and CCW torsional nystagmus appears in the primary position. (b) Right-beating, upbeating, and CW torsional nystagmus emerges during rightward gaze. (c) Left-beating, downbeating, and CCW torsional nystagmus develops during leftward gaze.
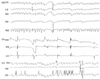
Figure 4
Oculography of patient 3. (a) Continuous horizontal pendular nystagmus appears in the primary position. (b) Right-beating nystagmus with a downbeat component develops during rightward gaze. (c) Nystagmus is suppressed during convergence.

Figure 6
Oculography of patient 4. (a) Spontaneous nystagmus is left beating in the primary position with fixation. (b) The spontaneous left-beating nystagmus changes to right-beating without fixation. (c) Optokinetic nystagmus is reversed.

References
1. Leigh RJ, Zee DS. Diagnosis of central disorders of ocular motility. The neurology of eye movements. 4th edn. New York: Oxford University Press.
2. Hoffmann S, Becker A, Hoerle S, Metz A, Oertel WH, Sommer N, et al. Autosomal dominant congenital nystagmus is not linked to 6p12, 7p11, and 15q11 in a German family. Am J Ophthalmol. 2004. 138:439–443.

3. Gelbart SS, Hoyt CS. Congenital nystagmus: a clinical perspective in infancy. Graefes Arch Clin Exp Ophthalmol. 1988. 226:178–180.

4. Leitch RJ, Thompson D, Harris CM, Chong K, Russell-Eggitt I, Kriss A. Achiasmia in a case of midline craniofacial cleft with seesaw nystagmus. Br J Ophthalmol. 1996. 80:1023–1024.

5. Migeon BR, Kennedy JF. Evidence for the inactivation of an X chromosome early in the development of the human female. Am J Hum Genet. 1975. 27:233–239.

6. Schneiderman LJ, Bartnof HS, Worthen DM. X-linked congenital nystagmus: a problem in genetic counseling. Ann Ophthalmol. 1976. 8:444–446.
7. Kerrison JB, Koenekoop RK, Arnould VJ, Zee D, Maumenee IH. Clinical features of autosomal dominant congenital nystagmus linked to chromosome 6p12. Am J Ophthalmol. 1998. 125:64–70.

8. Rosenblum SF, Rosenblum JA. Sex linked hereditary nystagmus. Metab Pediatr Syst Ophthalmol. 1987. 10:103–106.
9. Kerrison JB, Vagefi MR, Barmada MM, Maumenee IH. Congenital motor nystagmus linked to Xq26-q27. Am J Hum Genet. 1999. 64:600–607.

10. Forssman B. Hereditary studies of congenital nystagmus in a Swedish population. Ann Hum Genet. 1971. 35:119–138.

11. Kim JS, Ahn KW, Moon SY, Choi KD, Park SH, Koo JW. Isolated perverted head-shaking nystagmus in focal cerebellar infarction. Neurology. 2005. 64:575–576.

12. Han BI, Oh HJ, Kim JS. Nystagmus while recumbent in horizontal canal benign paroxysmal positional vertigo. Neurology. 2006. 66:706–710.

13. Abadi RV, Dickinson CM. Waveform characteristics in congenital nystagmus. Doc Ophthalmol. 1986. 64:153–167.

14. Jung R, Kornhuber HH. Results of electronystagmography in man: the value of optokinetic, vestibular, and spontaneous nystagmus for neurologic diagnosis and research. The oculomotor system. 1964. New York: Harper and Row;428–488. chap 19.
15. Dell'Osso LF, Weissman BM, Leigh RJ, Abel LA, Sheth NV. Hereditary congenital nystagmus and gaze-holding failure: the role of the neural integrator. Neurology. 1993. 43:1741–1749.
16. Bedell HE, Bollenbacher MA. Perception of motion smear in normal observers and in persons with congenital nystagmus. Invest Ophthalmol Vis Sci. 1996. 37:188–195.
17. Cogan DG. Congenital nystagmus. Can J Ophthalmol. 1967. 2:4–10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download