Small cortical strokes can produce isolated motor paresis that mimics peripheral neuropathy in the upper extremities. Several cases of isolated hand or finger paresis caused by small cortical infarctions have been reported.1-3 However, isolated shoulder weakness resulting from cortical infarction is extremely rare. Komatsu et al. reported a case of isolated shoulder paresis due to a small cortical infarction, but they did not report on the motor powers of individual muscles.4 We report here a patient presenting with isolated shoulder weakness caused by a small cortical infarction, whose individual muscles were evaluated in detail.
CASE REPORT
A 70-year-old man visited the emergency room complaining of sudden-onset general weakness just after napping. On physical examination we found that he could not lift his left arm above his head. He had been treated for hypertension and diabetes mellitus for the previous 20 years. He was known to have arrhythmia but was not taking anticoagulation medication. On the initial neurological examination he was alert and had no aphasia or agnosia. Cranial nerve examination revealed no facial weakness, dysarthria, or dysphagia. A thorough motor examination on his left arm revealed that the trapezius, deltoid, supraspinatus, and infraspinatus muscles were weak (Medical Research Council grade IV). However, the motor power of the sternocleidomastoid, biceps, triceps, wrist, and finger muscles were within normal limits. Sensations were perceived as normal in all modalities. Cortical sensory function, including stereognosis and two-point discrimination, tested on his hands, were normal. Deep tendon reflexes and cerebellar function tests were also normal.
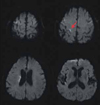
The result of his electrocardiogram showed atrial fibrillation. A brain magnetic resonance imaging (MRI) scan taken 4 hours after the onset showed scattered acute infarcts in the right frontotemporal area and periventricular deep white matter (Fig. 1). A magnetic resonance angiogram showed no steno-occlusive lesions in the intracranial and extracranial arteries. Transthoracic and transesophageal echocardiograms revealed vigorous spontaneous echogenic contrast with sludge in the left atrial appendage. Heparin was administered to the patient, which was switched to warfarin 3 days later. The weakness of the shoulder gradually improved and the patient could lift his left arm above his head 10 days later.
DISCUSSION
Examination of the individual muscles of the left arm in this patient revealed a discrepancy in muscle strength between the sternocleidomastoid and trapezius muscles, which are both innervated by the accessory nerve. In addition, our patient had weakness of the deltoid, supraspinatus, and infraspinatus muscles, which are each innervated by different nerves. These findings could not be explained by peripheral neuropathy, plexopathy, or radiculopathy. Therefore, a lesion in the motor cortex was considered to be responsible for the patient's symptom. Diffusion-weighted MRI revealed a small cortical lesion in the right precentral gyrus. This region is located just medial to the precentral knob, considered a motor hand area,5 and is correlated with the shoulder area in the motor homunculus.6 The concomitant infarcts in the right frontoparietal lobe were not considered to be related to the shoulder weakness. The discrepancy in muscle strength between the sternocleidomastoid and trapezius muscles might be due to sparing of the neck area in the motor homunculus.6-8
Our opinion was not supported by the electrodiagnostic studies, which is the limitation of this report. However, we believe that the sudden onset of weakness, the results of the individual muscle examinations, the gradual improvement of the weakness, and the associated acute lesion in the motor cortex were sufficient to exclude the possibility of peripheral nerve diseases.
The part of the body carrying the most delicate movements have, in general, the largest cortical representation.6 Thus, the motor shoulder area occupies a relatively small area in the motor homunculus. This may explain why isolated shoulder paresis is rare compared with isolated finger or hand weakness.
To the best of our knowledge, this case is only the second report of isolated shoulder weakness caused by a small cortical infarction.4 The present results suggest that when presented with isolated shoulder weakness, stroke should be considered in addition to peripheral neuropathies as a differential diagnosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download