Abstract
Background and Purpose
The mechanism of upbeat nystagmus is unknown and clinicoanatomical correlative studies in series of patients with upbeat nystagmus are limited.
Methods
Fifteen patients with upbeat nystagmus received full neuro-ophthalmological evaluation by the senior author. Nystagmus was observed using video Frenzel goggles and recorded with video-oculography. Brain lesions were documented with MRI.
Results
Lesions responsible for nystagmus were found throughout the brainstem, mainly in the paramedian area: in the medulla (n=8), pons (n=3), pons and midbrain with or without cerebellar lesions (n=3), and midbrain and thalamus (n=1). Underlying diseases comprised cerebral infarction (n=10), multiple sclerosis (n=2), cerebral hemorrhage (n=1), Wernicke encephalopathy (n=1), and hydrocephalus (n=1). Upbeat nystagmus was mostly transient and showed occasional evolution during the acute phase. In one patient with a bilateral medial medullary infarction, the upbeat nystagmus changed into a hemiseesaw pattern with near complete resolution of the unilateral lesion. Gaze and positional changes usually affected both the intensity and direction of the nystagmus. A patient with a cervicomedullary lesion showed a reversal of upbeat into downbeat nystagmus by straight-head hanging and leftward head turning while in the supine position. Gaze-evoked nystagmus (n=7), ocular tilt reaction (n=7), and internuclear ophthalmoplegia (n=4) were also commonly associated with upbeat nystagmus.
Upbeat nystagmus is a type of central vestibular nystagmus1 that is usually transient and is less common than downbeat nystagmus. Upbeat nystagmus usually increases with upward gaze and accompanies an impaired upward pursuit. Unlike downbeat nystagmus, upbeat nystagmus is usually not enhanced on lateral gaze and may evolve into downbeat nystagmus with convergence.
Upbeat nystagmus has been reported in patients with infarctions, hemorrhages, tumors, multiple sclerosis, Wernicke encephalopathy, epilepsy, brainstem encephalitis, Creutzfeldt-Jakob disease, Behcet syndrome, meningitis, Chiari malformation, and cerebellar degeneration.1-3 Transient or paroxysmal upbeat nystagmus may be found in individuals experiencing tobacco, barbiturate, and organophosphate intoxication, and after discontinuation of amitriptyline.4,5
Upbeat nystagmus occurs as a result of lesions in various locations and is often seen associated with pontomedullary and pontomesencehalic lesions.6 It has also been reported in patients with lesions in the anterior vermis of the cerebellum.7 In contrast to downbeat nystagmus, lesions of the paramedian brainstem are frequent in upbeat nystagmus.8
Several mechanisms have been proposed for upbeat nystagmus: (i) imbalance in the vertical vestibulo-ocular pathways, (ii) dysfunction of the neural integrator involved in vertical gaze holding, and (iii) impairment of the upward smooth pursuit. However, the exact mechanisms remain to be elucidated.1
There have been anecdotal reports on upbeat nystagmus. However, clinicoanatomical correlations have not been sought in series of patients.7,9 We report on the clinical characteristics of 15 patients with upbeat nystagmus and discuss the possible mechanisms based on the clinicoanatomical correlations.
A total of 15 patients were recruited at the Neuro-ophthalmology Clinic of Seoul National University Bundang Hospital from June 2003 to September 2005. The patients comprised six men and nine women ranging in age from 29 to 77 years (mean: 50.5 years). All patients received full neurological and neuro-ophthalmological evaluations by the senior author (J.S.K.). Some patients (patients 1 and 8-11) were reported on previously (Table 1).10-12
Upbeat nystagmus was defined only when the upbeat component predominated the horizontal and torsional ones in both eyes. Upbeat nystagmus that was either observed only during the vertical gaze or induced by positional changes was excluded from the analyses. Nystagmus was observed both with and without fixation using video Frenzel goggles (SLMED, Seoul, Korea). The effects of fixation, gaze, and positional changes were evaluated. In 11 patients, eye movements were recorded by video-oculography (SMI, Teltow, Germany).
All patients underwent MRI of the brain using a 1.5-T unit (Intera; Philips Medical Systems, Best, The Netherlands) with our standard imaging protocol (i.e., axial turbo spin-echo T2-weighted imaging, axial spin-echo T1-weighted imaging, and axial gradient- echo imaging). The imaging parameters were 4800/100 [repetition time (ms)/echo time (ms)] for T2-weighted imaging, 500/11 for T1-weighted imaging, and 700/23 for gradient-echo imaging with a section thickness of 3 mm, a matrix of 256×256 (interpolated to 512×512), and a field of view of 200-220 mm. Diffusion-weighted imaging was additionally performed in patients with acute infarctions with the following parameters: b=1000, 4119/89 (repetition time/echo time), a section thickness of 3 mm, a matrix of 128×128 (interpolated to 256×256), and a field of view of 220 mm.
Lesions responsible for nystagmus were found from the medulla to the thalamus (Table 1). Medullary lesions were the most common, and were observed in eight patients: four patients had medial medullary infarctions, two patients had lateral medullary infarctions, and two patients had dorsal lesions in the lower medulla (Fig. 1). Three patients showed lesions that were restricted to the pons, two patients had circumscribed lesions in the unilateral or bilateral pontine tegmentum, and one patient exhibited a diffuse infarction from the basis pontis to the tegmentum. One patient with brainstem infarction (patient 4) and another with Wernicke encephalopathy (patient 2) showed diffuse lesions involving both the pontine and midbrain tegmentum. In one patient with neurocysticercosis (patient 1), the midbrain and pons were compressed and displaced forward by hydrocephalus (Fig. 1). Compression of the midbrain by a hematoma was evident in a patient with a thalamic hemorrhage. Infarction was the most common cause of nystagmus, and was observed in 10 patients. Other causes included multiple sclerosis (n=2), hemorrhage (n=1), Wernicke encephalopathy (n=1), and hydrocephalus from neurocysticercosis (n=1).
Upbeat nystagmus primarily increased during either an upward or lateral gaze, and decreased during either a downward gaze or convergence. In two patients (patients 13 and 14), upbeat nystagmus primarily evolved into a horizontal one on downward gaze. In six patients, upbeat nystagmus was accompanied with a torsional component. In patient 9, who had a bilateral medial medullary infarction, upbeat nystagmus was associated with alternating horizontal components that resulted in a bow-tie pattern.10
In most patients, upbeat nystagmus was observed only during the acute stage of illness and resolved earlier than other ocular motor abnormalities. Only the patient (patient 14) who had multiple sclerosis in the cervicomedullary junction showed severe nystagmus that lasted at least several months (Fig. 2-A). In this patient, the upbeat nystagmus became a primarily torsional one with near complete resolution of the vertical component 5 months later (Fig. 2-B). Some patients showed an evolution of nystagmus during the acute stage. Horizontal nystagmus evolved into upbeat nystagmus in patient 13 with a lateral medullary infarction, and initial upbeat nystagmus evolved into downbeat and torsional nystagmus in patient 7 with bilateral pontine infarctions. In one patient (patient 9) with a bilateral medial medullary infarction, the upbeat nystagmus evolved into hemiseesaw nystagmus with a near-complete resolution of the unilateral lesion.10
The positional effects on upbeat nystagmus were studied thoroughly in patient 14. In this patient, upbeat nystagmus with a counterclockwise (from the patient's viewpoint) torsional component was suppressed by prone position, tilting the head to either side while sitting, and rightward head turning in the supine position. The nystagmus specifically reversed direction to downbeat and clockwise torsional components during straight-head hanging and leftward head turning while the patient was in the supine position (Fig. 3).
Most patients accompanied ocular motor abnormalities other than upbeat nystagmus that were specific to the underlying lesions. Gaze-evoked nystagmus (n=7), ocular tilt reaction (n=5), and internuclear ophthalmoplegia (n=4) were commonly observed as results of lesions throughout the brainstem. The pontine lesions induced various features of horizontal gaze palsy, including one-and-a-half syndrome, abducens palsy, and horizontal gaze palsy. Patients with medial medullary lesions experienced asymmetric gaze-evoked nystagmus, contralesional ocular tilt reaction, and ocular contrapulsion, while patients with lateral medullary lesions exhibited ipsilesional ocular tilt reaction, ocular ipsipulsion, and Horner syndrome. One patient with lower medullary lesions had ocular tilt reaction. The midbrain lesions, including thalamic hemorrhage that compressed the midbrain, gave rise to vertical gaze palsy either in isolation or with other signs of pretectal syndrome.
Our patients showed characteristic findings of upbeat nystagmus that were consistent with previous reports on this condition. In most of the patients, the nystagmus increased with upward gaze and decreased with both downward gaze and convergence. While lateral gaze usually increases or induces downbeat nystagmus, the effects of lateral gaze on upbeat nystagmus were variable, increasing nystagmus only in a small proportion of patients. Previously, the transition of upbeat to downbeat nystagmus with convergence was described in patients with Wernicke encephalopathy.13 However, none of our patients - including the one with Wernicke encephalopathy - showed such a transition, even though the intensity of the nystagmus decreased markedly with convergence in almost all of the patients.
The upbeat nystagmus in patient 14 was suppressed by positional changes in the direction of gravity, and reversed direction during straight-head hanging and leftward head turning while the patient was in the supine position. Positional modulation of upbeat nystagmus (i.e., upbeat in the supine position and downbeat in the prone position) was reported previously.14,15 Otolith function may influence vertical nystagmus, and gravity also plays an important role in vertical vestibular eye movements.16 Downbeat nystagmus is often increased in prone, supine, and upside-down positions.1,7,17,18 Downbeat nystagmus also may occur in healthy subjects with an upside-down head position19 or even when the head is simply no longer erect.20 In contrast, upbeat nystagmus is, at times, improved when the patient's head is upside-down, or even when the patient is in either the supine or the prone position.7,9,21,22 Upbeat nystagmus also occurs in normal subjects when the gravity load is artificially increased by centrifugation.23
In our patients, upbeat nystagmus was transient, lasting only several days or resolved within a month in most of them, while downbeat nystagmus usually persisted.1 Upbeat nystagmus also showed occasional evolution during the acute stage. These findings may reflect the central adaptation of the vestibulo-ocular system or the characteristics of the underlying diseases that were confined to the brainstem and tended to resolve over time. One of our patients who had a lateral medullary infarction showed evolution of upbeat nystagmus into downbeat nystagmus over several days during the acute stage. Cases of vertical nystagmus with spontaneous directional changes have rarely been described. Such a transition over several months had occurred previously in a patient with medial and posterior medullary hemorrhage.22 Another patient with Wernicke encephalopathy also showed an evolution of upbeat into downbeat nystagmus after one year, possibly due to baclofen treatment.24 Downbeat and upbeat nystagmus are the directional counterparts of a vestibular tone imbalance in the pitch plane. The close proximity of the areas that cause either upbeat or downbeat nystagmus in the medulla is consistent with occasional transitions between the two conditions.25,26
Our patients had various lesions, ranging from the lower medulla to the thalamus. Only one patient with Wernicke encephalopathy had a combined lesion in the cerebellar vermis. Previously, upbeat nystagmus has been known to arise from two separate intra-axial brainstem lesions in the tegmentum of either the pontomesencephalic or pontomedullary junction.21,25,27-29
Upbeat nystagmus may occur due to the disruption of the pathways involved in the upward vestibulo-ocular reflex (VOR) from the anterior semicircular canals to the ocular motor nuclei, or due to dysfunction of the neural integrator that is involved in vertical gaze holding. First of all, damage to the ascending excitatory projections from the bilateral anterior semicircular canals may lead to a downward drift of the eyes, giving rise to corrective upbeat nystagmus. In humans, signals from the anterior semicircular canals are mainly sent to the superior vestibular nucleus, which is connected to the oculomotor nucleus via the medial longitudinal fasciculus (MLF), and possibly via the ventral tegmental tract (VTT). In pontomesencephalic lesions, upbeat nystagmus may be explained by damage to the bilateral MLF or VTT. Indeed, internuclear ophthalmoplegia or ocular tilt reactions observed in most of our patients with pontomesencephalic lesions suggest MLF involvement. As the MLF contains both pathways from the contralateral anterior and posterior canals, various patterns of combined torsional and vertical nystagmus may develop in unilateral damage to the MLF, depending on the pathways involved.30 However, the induction of upbeat nystagmus by a MLF lesion requires selective or predominant damage to the bilateral pathways that are involved in the upward VOR, sparing the pathways involved in the downward VOR. This may explain why upbeat nystagmus is rarely observed in internuclear ophthalmoplegia.
The VTT may be another anatomical substrate for upbeat nystagmus. This tract originates in the superior vestibular nucleus, passes through the ventral pons, and transmits excitatory upward vestibular signals to the oculomotor nucleus.31-34 The VTT lies slightly ventral and lateral to the brachium conjunctivum in the lower pons, arching medially and decussating slightly above the level of the midpons, perhaps in the posterior part of the basis pontis in humans.8,34 This decussation in the midpons may explain why upbeat nystagmus may arise from a relatively small unilateral paramedian lesion in the upper level.8 However, in our patients with isolated pontine infarctions, the lesions primarily involved the dorsal pontine tegmentum.
In our study, the lesions responsible for nystagmus were most frequently located in the medulla. These medullary lesions can be divided into medial, lateral, and lower groups. In medial medullary infarctions, upbeat nystagmus was observed in four patients with either uni- or bilateral lesions. Occasional upbeat nystagmus during medial medullary infarction has been thought to involve the perihypoglossal nuclei, which consist of the nucleus prepositus hypoglossi, the nucleus of Roller, and the nucleus intercalatus.1 However, the evolution of upbeat into hemiseesaw nystagmus with the resolution of an unilateral lesion, as previously described in one of our patients with a bilateral infarction, suggests the involvement of the VOR pathways from both anterior semicircular canals as a mechanism of upbeat nystagmus.10 Because the MLF is a midline structure, the development of upbeat nystagmus in unilateral lesions may be attributable to damage to the decussating fibers from both anterior semicircular canals at the rostral medulla. Lateral medullary infarction, probably involving the vestibular nuclei, may generate various types of spontaneous nystagmus.
As previously reported,7,21,22,25,35-42 upbeat nystagmus resulted from caudal medullary lesions in two of our patients. As few anatomical structures are known to control vertical eye motion in this area, the mechanism of upbeat nystagmus from a caudal medullary lesion remains to be elucidated. Previously, the nuclei of intercalatus and Roller, which are caudal components of the perihypoglossal nuclei, have been proposed as anatomical substrates of upbeat nystagmus in this region. The nucleus of Roller receives a strong projection from the superior vestibular nucleus,43 and projects strongly to the flocculus44 via a tract that could be inhibitory.45 Accordingly, damage to the nucleus of Roller itself or to its afferent and efferent fibers may induce inhibition of the upward VOR pathway and upbeat nystagmus by disinhibiting the inhibitory flocculovestibular neurons. Indeed, the exponential decrease in the slow-phase velocity in one of our patients with a lower medullary lesion suggests the dysfunction of the neural integrator. The caudal cell groups of the paramedian tract, which are involved in the processing of vertical eye position through their projections to the cerebellar flocculus, may be another neural substrate for upbeat nystagmus in medial medullary infarction.46 Those cells are located near the nuclei of both Roller and intercalatus, receive afferent signals from the vestibular structures, and project to the flocculus.47
Two patients with midbrain lesions showed upbeat nystagmus with vertical gaze palsy and one of whom had typical findings of the pretectal syndrome. The interstitial nucleus of Cajal (INC) resides in the midbrain and subserves gaze holding in both the vertical and torsional planes. In unilateral lesions of the INC, ipsilesionally beating torsional nystagmus is combined with upbeat nystagmus in the contralesional eye.48 Bilateral damage to the INC may generate both vertical gaze palsy and upbeat nystagmus.
Figures and Tables
Figure 1
Brain MRIs of patients with upbeat nystagmus reveal various lesions from the lower medulla to the thalamus.
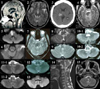
Figure 2
Video-oculography in patient 14 reveals conjugate upbeat nystagmus with an initial counterclockwise torsional component (A), which becomes mainly a torsional component with near-complete resolution of the vertical component 5 months later (B). Upbeat nystagmus shows a decelerating slow phase.
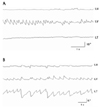
Figure 3
Positional modulation of upbeat nystagmus in patient 14. Upbeat nystagmus reverses direction to downbeat on straight-head hanging.

References
1. Leigh RJ, Zee DS. The neurology of eye movements. 1999. 3rd edn. New York: Oxford University Press.
2. Kumar A, Patni AH, Charbel F. The Chiari I malformation and the neurotologist. Otol Neurotol. 2002. 23:727–735.

3. Zingler VC, Strupp M, Jahn K, Glaser M, Herberger S, Kretzschmar HA, et al. Upbeat nystagmus as the initial clinical sign of Creutzfeldt-Jakob disease. Ann Neurol. 2005. 57:607–608.

4. Jay WM, Marcus RW, Jay MS. Primary position upbeat nystagmus with organophosphate poisoning. J Pediatr Ophthalmol Strabismus. 1982. 19:318–319.

5. Sibony PA, Evinger C, Manning KA. Tobacco-induced primary-position upbeat nystagmus. Ann Neurol. 1987. 21:53–58.
7. Baloh RW, Yee RD. Spontaneous vertical nystagmus. Rev Neurol (Paris). 1989. 145:527–532.
8. Pierrot-Deseilligny C, Milea D. Vertical nystagmus: clinical facts and hypotheses. Brain. 2005. 128:1237–1246.

9. Hirose G, Kawada J, Tsukada K, Yoshioka A, Sharpe JA. Upbeat nystagmus: clinicopathological and pathophysiological considerations. J Neurol Sci. 1991. 105:159–167.

10. Choi KD, Jung DS, Park KP, Jo JW, Kim JS. Bowtie and upbeat nystagmus evolving into hemi-seesaw nystagmus in medial medullary infarction: possible anatomic mechanisms. Neurology. 2004. 62:663–665.

11. Kim JS, Han DH, Moon SY, Park SH. Convergence-related nystagmus in a patient with hydrocephalus from shunt malfunction. J Korean Neurol Assoc. 2004. 22:392–395.
12. Kim JS, Choi KD, Oh SY, Park SH, Han MK, Yoon BW. Medial medullary infarction: abnormal ocular motor findings. Neurology. 2005. 65:1294–1298.

13. Cox TA, Corbett JJ, Thompson HS, Lennarson L. Upbeat nystagmus changing to downbeat nystagmus with convergence. Neurology. 1981. 31:891–892.

14. Mizuno M, Kudo Y, Yamane M. Upbeat nystagmus influenced by posture: report of two cases. Auris Nasus Larynx. 1990. 16:215–221.

15. Sakuma A, Kato I, Ogino S, Okada T, Takeyama I. Primary position upbeat nystagmus with special reference to alteration to downbeat nystagmus. Acta Otolaryngol Suppl. 1996. 522:43–46.
17. Halmagyi GM, Rudge P, Gresty MA, Sanders MD. Downbeating nystagmus. A review of 62 cases. Arch Neurol. 1983. 40:777–784.
18. Marti S, Palla A, Straumann D. Gravity dependence of ocular drift in patients with cerebellar downbeat nystagmus. Ann Neurol. 2002. 52:712–721.

19. Leigh RJ, Das VE, Seidman SH. A neurobiological approach to acquired nystagmus. Ann N Y Acad Sci. 2002. 956:380–390.

20. Goltz HC, Irving EL, Steinbach MJ, Eizenman M. Vertical eye position control in darkness: orbital position and body orientation interact to modulate drift velocity. Vision Res. 1997. 37:789–798.

21. Fisher A, Gresty M, Chambers B, Rudge P. Primary position upbeating nystagmus. A variety of central positional nystagmus. Brain. 1983. 106:949–964.
22. Janssen JC, Larner AJ, Morris H, Bronstein AM, Farmer SF. Upbeat nystagmus: clinicoanatomical correlation. J Neurol Neurosurg Psychiatry. 1998. 65:380–381.

23. Marcus JT, Bles W, Van Holten CR. Influence of gravitoinertial force on vestibular nystagmus in man observed in a centrifuge. Adv Space Res. 1989. 9:213–222.

24. Kastrup O, Maschke M, Keidel M, Diener HC. Presumed pharmacologically induced change from upbeat to downbeat nystagmus in a patient with Wernicke's encephalopathy. Clin Neurol Neurosurg. 2004. 107:70–72.

25. Keane JR, Itabashi HH. Upbeat nystagmus: clinicopathologic study of two patients. Neurology. 1987. 37:491–494.

26. Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex. Ann Neurol. 1994. 36:337–347.

27. Ranalli PJ, Sharpe JA. Upbeat nystagmus and the ventral tegmental pathway of the upward vestibulo-ocular reflex. Neurology. 1988. 38:1329–1330.

28. Kattah JC, Kolsky MP, Guy J, O' Doherty D. Primary position vertical nystagmus and cerebellar ataxia. Arch Neurol. 1983. 40:310–314.

29. Nakada T, Remler MP. Primary position upbeat nystagmus. Another central vestibular nystagmus? J Clin Neuroophthalmol. 1981. 1:185–189.
30. Oh K, Chang JH, Park KW, Lee DH, Choi KD, Kim JS. Jerky seesaw nystagmus in isolated internuclear ophthalmoplegia from focal pontine lesion. Neurology. 2005. 64:1313–1314.

31. Carpenter MB, Cowie RJ. Connections and oculomotor projections of the superior vestibular nucleus and cell group 'y'. Brain Res. 1985. 336:265–287.

32. Sato Y, Kawasaki T. Target neurons of floccular caudal zone inhibition in Y-group nucleus of vestibular nuclear complex. J Neurophysiol. 1987. 57:460–480.

33. Sato Y, Kawasaki T, Mizukoshi K. Eye movement control by Purkinje cell/climbing fiber zones of cerebellar flocculus in cat. Acta Otolaryngol Suppl. 1991. 481:237–241.

34. Uchino Y, Sasaki M, Isu N, Hirai N, Imagawa M, Endo K. Second-order vestibular neuron morphology of the extra-MLF anterior canal pathway in the cat. Exp Brain Res. 1994. 97:387–396.

35. Gilman N, Baloh RW, Tomiyasu U. Primary position upbeat nystagmus. A clinicopathologic study. Neurology. 1977. 27:294–298.

36. Kato I, Nakamura T, Watanabe J, Harada K, Aoyagi M, Katagiri T. Primary position upbeat nystagmus. Localizing value. Arch Neurol. 1985. 42:819–821.
37. Munro NA, Gaymard B, Rivaud S, Majdalani A, Pierrot-Deseilligny C. Upbeat nystagmus in a patient with a small medullary infarct. J Neurol Neurosurg Psychiatry. 1993. 56:1126–1128.

38. Hirose G, Ogasawara T, Shirakawa T, Kawada J, Kataoka S, Yoshioka A, et al. Primary position upbeat nystagmus due to unilateral medial medullary infarction. Ann Neurol. 1998. 43:403–406.

39. Ohkoshi N, Komatsu Y, Mizusawa H, Kanazawa I. Primary position upbeat nystagmus increased on downward gaze: clinicopathologic study of a patient with multiple sclerosis. Neurology. 1998. 50:551–553.

40. Tilikete C, Hermier M, Pelisson D, Vighetto A. Saccadic lateropulsion and upbeat nystagmus: disorders of caudal medulla. Ann Neurol. 2002. 52:658–662.

41. Tyler KL, Sandberg E, Baum KF. Medical medullary syndrome and meningovascular syphilis: a case report in an HIV-infected man and a review of the literature. Neurology. 1994. 44:2231–2235.

42. Minagar A, Sheremata WA, Tusa RJ. Perverted head-shaking nystagmus: a possible mechanism. Neurology. 2001. 57:887–889.

43. McCrea RA, Strassman A, Highstein SM. Anatomical and physiological characteristics of vestibular neurons mediating the vertical vestibulo-ocular reflexes of the squirrel monkey. J Comp Neurol. 1987. 264:571–594.

44. Langer T, Fuchs AF, Scudder CA, Chubb MC. Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1985. 235:1–25.

45. Büttner-Ennever JA, Büttner U. Büttner-Ennever JA, editor. The reticular formation. Neuroanatomy of the oculomotor system. 1988. Amsterdam: Elsevier;119–176.
46. Büttner-Ennever JA, Horn AK. Pathways from cell groups of the paramedian tracts to the floccular region. Ann N Y Acad Sci. 1996. 781:532–540.

47. Büttner-Ennever JA, Horn AKE, Schmidtke K. Cell groups of the medial longitudinal fasciculus and paramedian tracts. Rev Neurol (Paris). 1989. 145:533–539.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download