1. Armstrong DG, Boulton AJ, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017; 376:2367–2375. PMID:
28614678.

2. Raspovic KM, Wukich DK. Self-reported quality of life and diabetic foot infections. J Foot Ankle Surg. 2014; 53:716–719. PMID:
25128305.

3. Korean Diabetes Association. Diabetes fact sheet in Korea 2016. Assessed 1 December 2017. Available at:
http://www.diabetes.or.kr/.
4. Bae JI, Won JH, Kim JS, Kim MD, Yoon CJ, Cho YK. Prevalence and current status of treatment of diabetic foot in South Korea. J Korean Soc Radiol. 2016; 74:169–176.

5. Chung CH, Kim DJ, Kim J, Kim H, Kim H, Min KW, Park SW, Park JH, Baik SH, Son HS, Ahn CW, Oh JY, Lee S, Lee J, Choi KM, Choi I, Park IB. Current status of diabetic foot in Korean patients using national health insurance database. J Korean Diabetes Assoc. 2006; 30:372–376.

6. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012; 54:e132–73. PMID:
22619242.
7. Peters EJ, Lipsky BA. Diagnosis and management of infection in the diabetic foot. Med Clin North Am. 2013; 97:911–946. PMID:
23992901.

8. Nather A, Bee CS, Huak CY, Chew JL, Lin CB, Neo S, Sim EY. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008; 22:77–82. PMID:
18280436.

9. Uckay I, Gariani K, Pataky Z, Lipsky BA. Diabetic foot infections: state-of-the-art. Diabetes Obes Metab. 2014; 16:305–316. PMID:
23911085.

10. Miyan Z, Fawwad A, Sabir R, Basit A. Microbiological pattern of diabetic foot infections at a tertiary care center in a developing country. J Pak Med Assoc. 2017; 67:665–669. PMID:
28507348.
11. Lipsky BA, Aragón-Sánchez J, Diggle M, Embil J, Kono S, Lavery L, Senneville É, Urbančič-Rovan V, Van Asten S.
International Working Group on the Diabetic Foot,
Peters EJ. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016; 32(Suppl 1):45–74. PMID:
26386266.
12. Korean Society for Diabetic Foot. Korean guideline for management of diabetic foot. 1st ed. Seoul: Koonja Press;2014. p. 49–66.
13. Lipsky BA, Pecoraro RE, Larson SA, Hanley ME, Ahroni JH. Outpatient management of uncomplicated lower-extremity infections in diabetic patients. Arch Intern Med. 1990; 150:790–797. PMID:
2183732.

14. Young H, Miller W, Burnham R, Heard S, Berg C, Jenkins TC. How do preoperative antibiotics affect culture yield in diabetic foot infections? Open Forum Infect Dis. 2017; 4:ofx016. PMID:
28480287.

15. Chakraborti C, Le C, Yanofsky A. Sensitivity of superficial cultures in lower extremity wounds. J Hosp Med. 2010; 5:415–420. PMID:
20845440.

16. Pellizzer G, Strazzabosco M, Presi S, Furlan F, Lora L, Benedetti P, Bonato M, Erle G, de Lalla F. Deep tissue biopsy vs. superficial swab culture monitoring in the microbiological assessment of limb-threatening diabetic foot infection. Diabet Med. 2001; 18:822–827. PMID:
11678973.

17. Nelson EA, Wright-Hughes A, Brown S, Lipsky BA, Backhouse M, Bhogal M, Ndosi M, Reynolds C, Sykes G, Dowson C, Edmonds M, Vowden P, Jude EB, Dickie T, Nixon J. Concordance in diabetic foot ulceration: a cross-sectional study of agreement between wound swabbing and tissue sampling in infected ulcers. Health Technol Assess. 2016; 20:1–176.

18. Rhee SM, Han SK, Kim WK. Difference of microbiology according to tissue sampling in diabetic ulcers. J Korean Soc Plast Reconstr Surg. 2010; 37:1–6.
19. Senneville E, Melliez H, Beltrand E, Legout L, Valette M, Cazaubiel M, Cordonnier M, Caillaux M, Yazdanpanah Y, Mouton Y. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures. Clin Infect Dis. 2006; 42:57–62. PMID:
16323092.

20. Embil JM, Trepman E. Microbiological evaluation of diabetic foot osteomyelitis. Clin Infect Dis. 2006; 42:63–65. PMID:
16323093.
21. Rhee SM, Han SK, Kim WK. Difference of microbiology according to tissue sampling in bone involved diabetic ulcers. J Korean Soc Plast Reconstr Surg. 2010; 37:335–339.
22. Sotto A, Richard JL, Combescure C, Jourdan N, Schuldiner S, Bouziges N, Lavigne JP. Beneficial effects of implementing guidelines on microbiology and costs of infected diabetic foot ulcers. Diabetologia. 2010; 53:2249–2255. PMID:
20571753.

23. Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis. 2008; 47:1537–1545. PMID:
18990064.

24. Lipsky BA, Pecoraro RE, Wheat LJ. The diabetic foot. Soft tissue and bone infection. Infect Dis Clin North Am. 1990; 4:409–432. PMID:
2212597.
25. Saseedharan S, Sahu M, Chaddha R, Pathrose E, Bal A, Bhalekar P, Sekar P, Krishnan P. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. [Epub ahead of print]. Braz J Microbiol. 2017.

26. Hatipoglu M, Mutluoglu M, Uzun G, Karabacak E, Turhan V, Lipsky BA. The microbiologic profile of diabetic foot infections in Turkey: a 20-year systematic review: diabetic foot infections in Turkey. Eur J Clin Microbiol Infect Dis. 2014; 33:871–878. PMID:
24452966.
27. Ramakant P, Verma AK, Misra R, Prasad KN, Chand G, Mishra A, Agarwal G, Agarwal A, Mishra SK. Changing microbiological profile of pathogenic bacteria in diabetic foot infections: time for a rethink on which empirical therapy to choose? Diabetologia. 2011; 54:58–64. PMID:
20835702.

28. Song JY. Antimicrobial therapy in diabetic foot infections. J Korean Diabetes. 2011; 12:83–87.

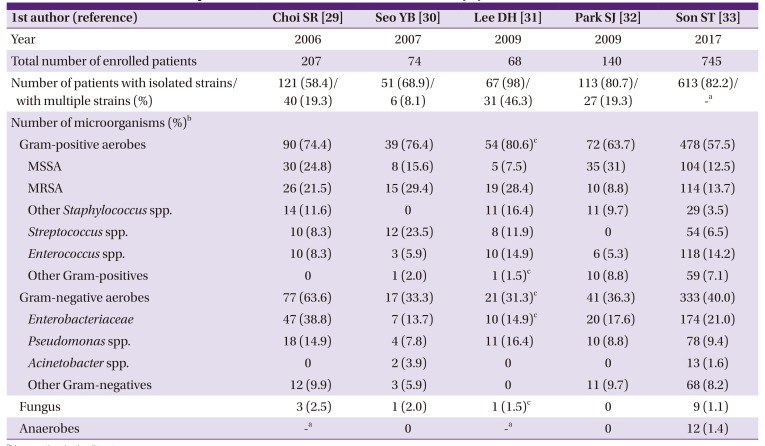
29. Choi SR, Lee CK, Kim DW, Han SK, Kim WK. Bacteriology and antibiotic sensitivity for diabetic foot ulcer. J Korean Soc Plast Reconstr Surg. 2006; 33:330–334.
30. Seo YB, Noh JY, Huh JY, Lee J, Song JY, Han SK, Kim WJ, Cheong HJ. Diabetic foot infections: microbiology analysis based on deep tissue biopsy. Infect Chemother. 2007; 39:237–242.
31. Lee DH, Han SH, Park MJ. Analysis of initial choice antibiotics efficacy in diabetic foot infection. J Korean Foot Ankle Soc. 2009; 13:146–149.
32. Park SJ, Jung HJ, Shin HK, Kim E, Lim JJ, Yoon JW. Microbiology and antibiotic selection for diabetic foot infections. J Korean Foot Ankle Soc. 2009; 13:150–155.
33. Son ST, Han SK, Lee TY, Namgoong S, Dhong ES. The microbiology of diabetic foot infections in Korea. J Wound Management Res. 2017; 13:8–12.

34. Charles PG, Uckay I, Kressmann B, Emonet S, Lipsky BA. The role of anaerobes in diabetic foot infections. Anaerobe. 2015; 34:8–13. PMID:
25841893.

35. Lavigne JP, Sotto A, Dunyach-Remy C, Lipsky BA. New molecular techniques to study the skin microbiota of diabetic foot ulcers. Adv Wound Care (New Rochelle). 2015; 4:38–49. PMID:
25566413.

36. Stappers MH, Hagen F, Reimnitz P, Mouton JW, Meis JF, Gyssens IC.
Bacteroides fragilis in biopsies of patients with major abscesses and diabetic foot infections: direct molecular versus culture-based detection. Diagn Microbiol Infect Dis. 2016; 85:263–265. PMID:
27112830.
37. Malone M, Gosbell IB, Dickson HG, Vickery K, Espedido BA, Jensen SO. Can molecular DNA-based techniques unravel the truth about diabetic foot infections? Diabetes Metab Res Rev. 2017; 33:e2834.

38. Eleftheriadou I, Tentolouris N, Argiana V, Jude E, Boulton AJ. Methicillin-resistant
Staphylococcus aureus in diabetic foot infections. Drugs. 2010; 70:1785–1797. PMID:
20836573.
39. Lee JS, Son ST, Han SK. Risk factors of methicillin-resistant Staphylococcus aureus and Pseudomonas infection in diabetic foot ulcers in Korea. J Wound Management Res. 2017; 13:29–34.
40. Rolain JM, Abat C, Brouqui P, Raoult D. Worldwide decrease in methicillin-resistant
Staphylococcus aureus: do we understand something? Clin Microbiol Infect. 2015; 21:515–517. PMID:
25941171.
41. Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S; Emerging Infections Program–Active Bacterial Core Surveillance MRSA Surveillance Investigators. National burden of invasive methicillin-resistant
Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013; 173:1970–1978. PMID:
24043270.
42. Landrum ML, Neumann C, Cook C, Chukwuma U, Ellis MW, Hospenthal DR, Murray CK. Epidemiology of
Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA. 2012; 308:50–59. PMID:
22760291.

43. Fatkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant
Staphylococcus aureus: sense, nonsense, and evidence. Lancet. 2015; 385:1146–1149. PMID:
25150745.
44. Zenelaj B, Bouvet C, Lipsky BA, Uckay I. Do diabetic foot infections with methicillin-resistant
Staphylococcus aureus differ from those with other pathogens? Int J Low Extrem Wounds. 2014; 13:263–272. PMID:
25288579.
45. Reveles KR, Duhon BM, Moore RJ, Hand EO, Howell CK. Epidemiology of methicillin-resistant
Staphylococcus aureus diabetic foot infections in a large academic hospital: implications for antimicrobial stewardship. PLoS One. 2016; 11:e0161658. PMID:
27556897.
46. Kwak YG, Kim NJ, Choi SH, Choi SH, Chung JW, Choo EJ, Kim KH, Yun NR, Lee S, Kwon KT, Cho JH. Clinical characteristics and organisms causing erysipelas and cellulitis. Infect Chemother. 2012; 44:45–50.

47. Choi SH, Choi SH, Kwak YG, Chung JW, Choo EJ, Kim KH, Yun NR, Lee S, Kwon KT, Choi JH, Kim NJ. Clinical characteristics and causative organisms of community-acquired necrotizing fasciitis. Infect Chemother. 2012; 44:180–184.

48. Park SY, Kim T, Choi SH, Jung J, Yu SN, Hong HL, Kim YK, Park SY, Song EH, Park KH, Cho OH, Choi SH, Kwak YG. The Korean SSTI (Skin and Soft Tissue Infection) study group. A multicenter study of clinical features and organisms causing community-onset cellulitis in Korea. [abstract]. Int J Antimicrob Agents. 2017; 50(Suppl 1):S75.
49. Kim T, Park SY, Kwak YG, Choi SH, Jung J, SN Y, Hong HL, Kim YK, Park SY, Song EH, Park KH, Cho OH, Choi SH. The Korean SSTI (Skin and Soft Tissue Infection) study group. A multicenter study of clinical characteristics and microbial etiology in community-onset necrotizing fasciitis in Korea [abstract]. Int J Antimicrob Agents. 2017; 50(Suppl 1):S136.
50. Rastogi A, Sukumar S, Hajela A, Mukherjee S, Dutta P, Bhadada SK, Bhansali A. The microbiology of diabetic foot infections in patients recently treated with antibiotic therapy: a prospective study from India. J Diabetes Complications. 2017; 31:407–412. PMID:
27894749.

51. Hatipoglu M, Mutluoglu M, Turhan V, Uzun G, Lipsky BA;, Sevim E, Demiraslan H, Eryilmaz E, Ozuguz C, Memis A, Ay H, Arda B, Uysal S, Motor VK, Kader C, Erturk A, Coskun O, Duygu F, Guler S, Altay FA, Ogutlu A, Bolukcu S, Yildiz S, Kandemir O, Aslaner H, Polat A, Karahocagil MK, Yasar KK, Sehmen E, Kilic S, Sunbul M, Gencer S, Bozkurt F, Yanik T, Oztoprak N, Batirel A, Sozen H, Kilic I, Celik I, Ay B, Tosun S, Kadanali A, Çomoglu S, Denk A, Hosoglu S, Aydin O, Elaldi N, Akalin S, Kandemir B, Akbulut A, Demirdal T, Balik R, Azak E, Sengoz G. Causative pathogens and antibiotic resistance in diabetic foot infections: a prospective multi-center study. J Diabetes Complications. 2016; 30:910–916. PMID:
26965794.

52. Anvarinejad M, Pouladfar G, Japoni A, Bolandparvaz S, Satiary Z, Abbasi P, Mardaneh J. Isolation and antibiotic susceptibility of the microorganisms isolated from diabetic foot infections in Nemazee Hospital, Southern Iran. J Pathog. 2015; 2015:328796. PMID:
26843987.

53. Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis. 2007; 44:562–565. PMID:
17243061.

54. Lipsky BA, Polis AB, Lantz KC, Norquist JM, Abramson MA. The value of a wound score for diabetic foot infections in predicting treatment outcome: a prospective analysis from the SIDESTEP trial. Wound Repair Regen. 2009; 17:671–677. PMID:
19671126.

55. Aragón-Sánchez J. Seminar review: a review of the basis of surgical treatment of diabetic foot infections. Int J Low Extrem Wounds. 2011; 10:33–65. PMID:
21444608.

56. Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004; 39(Suppl 2):S83–6. PMID:
15306984.

57. Edmonds M. Double trouble: infection and ischemia in the diabetic foot. Int J Low Extrem Wounds. 2009; 8:62–63. PMID:
19443892.

58. Richard JL, Lavigne JP, Sotto A. Diabetes and foot infection: more than double trouble. Diabetes Metab Res Rev. 2012; 28(Suppl 1):46–53. PMID:
22271723.

59. Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother. 2015; 16:821–832. PMID:
25736920.

60. Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs. 2009; 11:119–128. PMID:
19147524.

61. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015; CD009061. PMID:
26337865.

62. Lee S, Han SW, Kim KW, Song DY, Kwon KT. Third-generation cephalosporin resistance of community-onset
Escherichia coli and
Klebsiella pneumoniae bacteremia in a secondary hospital. Korean J Intern Med. 2014; 29:49–56. PMID:
24574833.
63. Kwak YG, Choi SH, Kim T, Park SY, Seo SH, Kim MB, Choi SH. Clinical guidelines for the antibiotic treatment for community-acquired skin and soft tissue infection. Infect Chemother. 2017; 49:301–325. PMID:
29299899.

64. Health Insurance Review and Asessment Service. Portal service for medical institution. Accessed 8 January 2018. Available at:
http://www.hira.or.kr/.
65. Uçkay I, Aragón-Sánchez J, Lew D, Lipsky BA. Diabetic foot infections: what have we learned in the last 30 years? Int J Infect Dis. 2015; 40:81–91. PMID:
26460089.

66. Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. Foreign body infections due to
Staphylococcus epidermidis
. Ann Med. 2009; 41:109–119. PMID:
18720093.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download